Leveraging Micro-CT Scanning to Analyze Parasitic Plant-Host Interactions
Summary
Micro-CT is a non-destructive tool that can analyze plant structures in three dimensions. The present protocol describes the sample preparation to leverage micro-CT to analyze parasitic plant structure and function. Different species are used to highlight the advantages of this method when coupled with specific preparations.
Abstract
Micro-CT scanning has become an established tool in investigating plant structure and function. Its non-destructive nature, combined with the possibility of three-dimensional visualization and virtual sectioning, has allowed novel and increasingly detailed analysis of complex plant organs. Interactions among plants, including between parasitic plants and their hosts, can also be explored. However, sample preparation before scanning becomes crucial due to the interaction between these plants, which often differ in tissue organization and composition. Furthermore, the broad diversity of parasitic flowering plants, ranging from highly reduced vegetative bodies to trees, herbs, and shrubs, must be considered during the sampling, treatment, and preparation of parasite-host material. Here two different approaches are described for introducing contrast solutions into the parasite and/or host plants, focusing on analyzing the haustorium. This organ promotes connection and communication between the two plants. Following a simple approach, details of haustorium tissue organization can be explored three-dimensionally, as shown here for euphytoid, vine, and mistletoe parasitic species. Selecting specific contrasting agents and application approaches also allow detailed observation of endoparasite spread within the host body and detection of direct vessel-to-vessel connection between parasite and host, as shown here for an obligate root parasite. Thus, the protocol discussed here can be applied to the broad diversity of parasitic flowering plants to advance the understanding of their development, structure, and functioning.
Introduction
High-resolution x-ray microcomputed tomography (micro-CT) is an imaging method in which multiple radiographs (projections) of a sample are recorded from different viewing angles and later used to provide a virtual reconstruction of the sample1. This virtual object can then be analyzed, manipulated, and segmented, allowing non-destructive exploration in three dimensions2. Initially designed for medical analyses and later for industrial applications, micro-CT also offers the advantage of visualizing inner organs and tissues without the need for invasive procedures3. Like other forms of imaging, micro-CT works with a trade-off between the field of view and pixel size, which means that high-resolution imaging of large samples is nearly unattainable4. Advances in using high-energy X-ray sources (i.e., synchrotron) and secondary optical magnification are constantly being made, allowing the smallest resolution to reach under 100 nm5,6. Nevertheless, longer scanning times are necessary for large samples, increasing the chance of artifacts due to sample movement or deformation inside the scanner. Furthermore, micro-CT is generally limited by natural density variations within the sample and how the sample interacts with X-rays. While a higher X-ray dose is best for penetrating denser samples, it is less efficient in capturing variations in density within and between the sample and its surrounding medium7. On the other hand, a lower X-ray dose offers less penetration power and often requires longer scanning times but more sensitivity in density detection7.
These restrictions have long hampered the use of microtomography for plant sciences, given that most plant tissues are composed of light (non-dense) tissue with low X-ray absorption8. The first applications of micro-CT were focused on mapping root networks within the soil matrix9,10. Later, plant structures with more significant differences in tissue density, such as wood, began to be explored. This has allowed investigations of xylem functionality11,12, development of complex tissue organizations13,14, and interactions among plants15,16,17. The analysis of soft and homogeneous tissue is becoming widespread due to contrast agents, which are now standard procedure in preparations for micro-CT scanning of plant samples. However, protocols for contrast introduction can have different results depending on sample volume, structural properties, and the type of solution used8. Ideally, the contrast agent should enhance distinction among different tissues, enable tissue/organ functionality evaluation, and/or provide biochemical information about a tissue18. Therefore, adequate sample treatment, preparation, and mounting before scanning become crucial for any micro-CT analysis.
Micro-CT of the parasitic plant haustorium
Parasitic flowering plants represent a distinct functional group of angiosperms characterized by an organ known as haustorium19. This multicellular organ, a developmental hybrid between a modified stem and a root, acts on the host's attachment, penetration, and contact by a parasite20. For this reason, the haustorium is considered to "embody the very idea of parasitism among plants"21. A detailed understanding of this organ's development, structure, and functioning is crucial for parasitic plant ecology, evolution, and management studies. Nevertheless, parasitic plants' overall complexity and highly modified structure and haustoria often hinder detailed analysis and comparison. Haustorium connections are also usually extensive and not homogenous in tissue and cell distribution (Figure 1). In this context, while working with small tissue fragments allows easier manipulation and higher resolution, it can lead to erroneous conclusions about the three-dimensional architecture of complex structures, such as the parasitic plant haustorium.
Although there is a vast literature on haustorium anatomy and ultrastructure for most parasitic plant species, the three-dimensional organization and the spatial relationship between parasite and host tissues remains poorly explored17. In a recent work by Masumoto et al.22, over 300 serial semi-thin microtome sections were imaged and reconstructed into a three-dimensional virtual object representing the haustorium of two parasite species. This method's excellent level of detail provides unprecedented insights into the haustorium's cellular and anatomical 3-D structure. However, such a time-consuming technique would forbid a similar analysis in parasites with more extensive haustorium connections. The use of micro-CT emerges as an excellent tool for three-dimensional analysis of complex and often bulky haustoria of parasitic plants. Although not a substitute for detailed anatomical sectioning and other complementary forms of microscopy analyses17,23, results obtained via micro-CT scanning, especially for large samples, can also serve as a guide to direct the sub-sampling of smaller segments, which can then be analyzed using other tools, such as confocal and electron microscopy, or re-analyzed with high-resolution micro-CT systems.
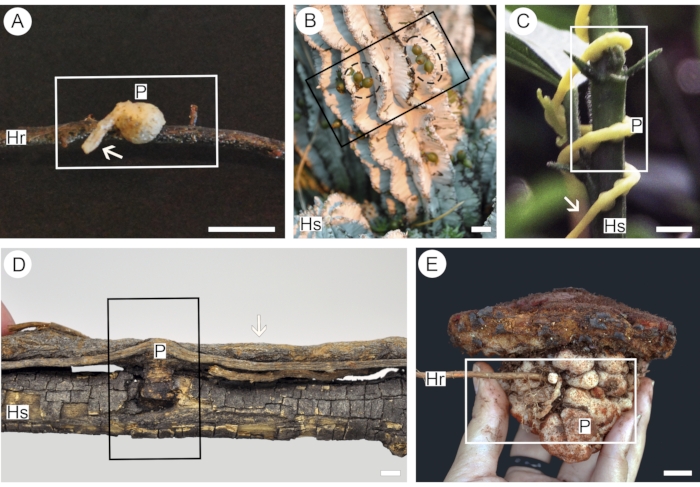
Figure 1: Parasitic plants of different functional groups used in this protocol. Euphytoid parasite Pyrularia pubera (A), endoparasite Viscum minimum (B) with green fruits (dashed black circle), parasitic vine Cuscuta americana (C), mistletoe Struthanthus martianus (D), obligate root parasite Scybalium fungiforme (E). Segments of the host root (Hr) or stem (Hs) facilitate the application of contrast into the parasite haustorium (P). The presence of parasite mother root/stem (arrows) in the sample allows analysis of haustorium vessel organization. Rectangles indicate segments of the sample used for analysis. Scale bars = 2 cm. Please click here to view a larger version of this figure.
As micro-CT becomes an increasingly popular technique in plant sciences, there are guides, protocols, and literature dealing with sample scanning, three-dimensional reconstruction, segmentation, and analysis3,10,24. Thus, these steps will not be discussed here. As with any imagining technique, appropriate treatment and mounting of samples are a fundamental, albeit often being an overlooked procedure. For this reason, this protocol focuses on the preparation of haustorium samples for micro-CT scanning. More specifically, this protocol describes two approaches for introducing contrast agents into haustorium samples to improve visualization of different tissues and cell types in the haustorium, to facilitate the detection of parasitic tissue within the host root/stem, and to analyze parasite-host vascular connections in three dimensions. The preparations described here can also be adapted to the analysis of other plant structures.
Five species were used to better illustrate the convenience of the protocol described here. Each species represents one of the five functional groups of parasitic flowering plants, thus addressing specific points related to the functionality of each group. Pyrularia pubera (Santalaceae) was chosen to represent euphytoid parasites, which germinate in the ground and form multiple haustoria that connect the parasite to the roots of its hosts25. The haustoria created by these plants are often tenuous and easily torn apart from the host26 (Figure 1A), thus requiring a more delicate handling process. Endoparasites are represented here by Viscum minimum (Viscaceae). Species in this functional group are only visible outside the body of their hosts for short periods (Figure 1B) and live most of their life cycles as significantly reduced and mycelial-like strands of cells embedded within host tissues25. A third functional group comprises parasitic vines, which germinate on the ground but form only rudimentary roots, relying on multiple haustoria that attach to the stems of host plants25 (Figure 1C). Here, this functional group is represented by Cuscuta americana (Convolvulaceae). Contrary to parasitic vines, mistletoes germinate directly upon the branches of their host plants and develop either multiple or solitary haustoria25. The species chosen to illustrate this functional group is Struthanthus martianus (Loranthaceae), which forms various connections with the host branch (Figure 1D). Analysis of solitary mistletoe haustoria using a combination of micro-CT and light microscopy can be found in Teixeira-Costa & Ceccantini17. Finally, obligate root parasites comprise species that germinate on the ground and penetrate the roots of host plants, upon which they are entirely dependent from the earliest growth stages25. These plants are represented here by Scybalium fungiforme (Balanophoraceae), which produce large tuber-like haustoria (Figure 1E).
All plant samples used in this protocol were fixed in a 70% formalin acetic acid alcohol (FAA 70). The fixation upon sampling is crucial for preserving plant tissues, especially if subsequent anatomical analyses are needed. In the case of parasitic plant haustorium, fixation is also essential, as this organ is often primarily composed of non-lignified parenchyma cells20. Detailed protocols for plant tissue fixation, including the preparation of fixative solutions, can be found elsewhere27. On the other hand, to a greater or lesser degree, fixatives can cause alterations of a sample's physical and chemical properties, rendering it unsuitable for specific biomechanical and histochemical analyses. Thus, fresh samples, i.e., non-fixated material collected immediately before preparation, can also be used with this protocol. Details on how to handle fresh samples and troubleshooting suggestions for fixated material are provided in the discussion section.
Protocol
1. Parasitic plant sample selection
- Collect the entire parasitic plant haustorium, including the attached host stem/root and segments of both proximal and distal ends of the parasitized host organ; the ideal length of each segment is equivalent to double the diameter of the haustorium.
NOTE: For lateral haustoria, include part of the parasite mother stem/root from which the haustorium was formed (Figure 1A,B,D). For endoparasites, collect a segment of the host stem/root in which signs of the parasite are visible (Figure 1B). In the case of terminal connections, the whole plant should be collected (Figure 1E). - Submerge the whole sample in fixative solution (e.g., FAA) in a volumetric proportion of 1:10 (sample: fixative). Leave samples in fixative for at least 1 day, depending on the size of the sample27.
NOTE: Samples can be stored in fixative before scanning or be transferred to a preserving solution (e.g., ethanol 70%). Fresh samples can also be used if the subsequent anatomical analysis is not warranted (see Discussion section). If working with fresh material, set up the apparatus for perfusion of the contrast solution, then collect the sample. The sample should not be allowed to dry out.
2. Application of contrasting solutions
- Chose the application method to be used. Use the vacuum method (step 2.3) for small (Figure 1A) or non-woody (Figure 1B) samples. Use the perfusion method for larger samples, provided it includes a segment of the host stem/root is (Figure 1A,C-E).
- Wear rubber gloves and other appropriate personal protection equipment (e.g., laboratory coat) when manipulating the sample regardless of the approach chosen in the following steps.
CAUTION: All contrasting solutions include heavy metal salts in their composition, and therefore should not be handled without adequate personal protection equipment and under a fume hood. - For the vacuum method, follow the steps mentioned below.
- Choose an appropriate vial and label it. The vial must be large enough to accommodate the sample and the contrasting solution, usually in a proportion of 1:10. Check instructions from the manufacturer to ensure that the vial can withstand low to moderate vacuum.
NOTE: Do not fill the vial to the brim, as the negative pressure (vacuum) can cause the liquid to spill. - Place the sample in the vial with the contrasting solution (1% iodine or 3% phosphotungstate, see Table of Materials). Then place the vial in a vacuum chamber or desiccator connected to a vacuum pump. Remove the lid from the vial, then close the vacuum chamber or desiccator.
- Check that there are no cracks on the vacuum chamber/desiccator and that the vacuum pump has enough oil.
- Close the exhaustion valve of the pump to prevent air from scaping and open the exhaustion valve of the chamber/desiccator to force the air out.
- Turn on the pump and wait until the pressure reaches approximately 20" Hg.
CAUTION: This process is usually fast, so do not leave the vacuum system unattended.
NOTE: While the metric system unit for pressure is Pascal (Pa), pressure gauges in most laboratory vacuum pumps display pressure in inches of mercury ("Hg), pound per square inch (psi), or bars. 20" Hg equals ca. 67.7 Pa, 10 psi, or 0.7 bar. - Close the chamber/desiccator exhaust valve to prevent air from re-entering, then quickly turn off the pump.
- Leave the sample under vacuum for at least 2 h; larger samples require longer for the contrasting solution to penetrate it.
- After the desired period, remove the sample from the contrasting solution to prepare it for scanning.
- Slowly open the chamber/desiccator exhaustion valve to allow air to enter it.
- Wait for the pressure in the chamber/desiccator to be fully exhausted (i.e., pressure gauge reaches near 0), then carefully open it to retrieve the sample.
- Discard the contrasting solution appropriately and keep the sample in preparation for scanning.
- Choose an appropriate vial and label it. The vial must be large enough to accommodate the sample and the contrasting solution, usually in a proportion of 1:10. Check instructions from the manufacturer to ensure that the vial can withstand low to moderate vacuum.
- For the perfusion method, follow the steps mentioned below.
- Select a supply tank for the contrasting solution according to the size of the sample. Use a 50 mL syringe (with no needle or plunger) for small samples (Figure 2A) or a 1 L intravenous medical kit for large samples (Figure 2B).
- Connect one end of a transparent plastic tubing (see Table of Materials) to the supply tank, then connect the other end to a two-way or three-way valve. Connect a second tubing to a different outlet in the valve (Figure 2A,B).
- Secure the supply tank at an elevated position without disassembling the apparatus set up in the previous step.
NOTE: The vertical distance between the tank and the sample will dictate the solution perfusion pressure (Figure 2B). A distance of 20-50 cm is enough for small samples. For large samples, a distance of 1 m is more adequate. - Close the three-way (Figure 2C) or two-way (Figure 2D) valve to prevent liquid from exiting the tubing system, then pour the contrasting solution into the supply tank. If using an intravenous medical kit, fill the bag with the solution and close the valve before securing the apparatus at an elevated position.
- Ensure that no large air bubbles are formed along the tubing system (Figure 2E). If necessary, allow the contrast solution to flow out of the tubing until the bubble is removed. Close the valve again and leave the apparatus in place.
- To prepare the sample for perfusion of the contrast solution, keep it submerged in liquid (water, ethanol, or fixative) and cut off the tip of the proximal end of the host stem/root in the sample (Figure 2F).
- Remove the sample from the liquid in which it was stored and wrap it in a paraffin film to avoid desiccation. Keep the sample nearby and ready to be connected to the apparatus.
- Carefully open the valve to allow the contrasting solution to flow slowly and fill the plastic tubing connected to the tank while holding the open end of the system at a slightly elevated position to prevent the contrasting solution from spilling. Again, ensure that no large air bubbles form along the tube.
- Connect the proximal end of the host stem/root in the sample to the open end of the tubing system (Figure 2B, magnified region). Avoid introducing air bubbles into the system during this step. If necessary, disconnect the sample from the apparatus and remove air bubbles from the system by allowing the solution to flow.
- While keeping the sample connected to the apparatus, place it inside a container to avoid leakage of the contrasting solution into the area where the experiment was set up. Use tubes of different diameters, plastic zip-ties, and valve adaptors (Figure 2G) to ensure all connections in the apparatus are well-fit, accommodate host branches of various sizes, and that the solution is not leaking out of the tubing system (Figure 2B, magnified region).
- Let the solution perfuse the sample for at least 2 h, or until the solution accumulates inside the container.
- Close the valve and carefully disconnect the sample from the apparatus. Drain the remainder of the solution into the container and dispose of it appropriately.
- Remove the paraffin film from the sample in preparation for scanning.
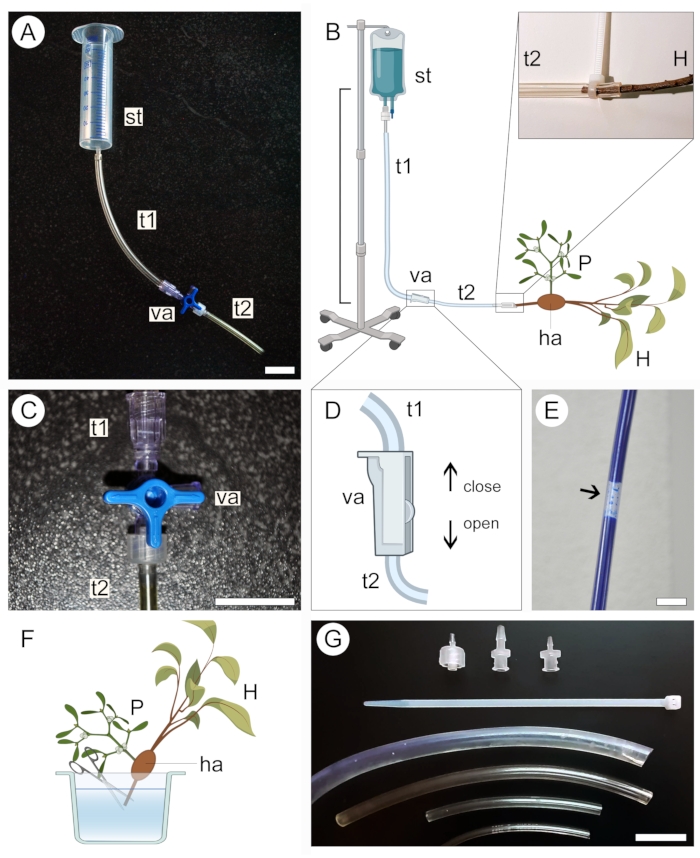
Figure 2: Perfusion approach for contrast application. Small (A) and large (B) versions of the perfusion apparatus include a supply tank (st) and two plastic tubes (t1 and t2) connected by a valve (va). The proximal end of the host stem (H) bearing a parasite (P) attached to it via the haustorium (ha) is connected to the open end of the system (B, expanded). A three-way (C) or a two-way (D) valve is used to help prevent the formation of air bubbles inside the tubing system, which block the passage of contrasting solution (E). The tip of the proximal end of the host stem (H) is cut underwater to allow passage of the contrast solution (F). Zip-ties, valve adaptors, and tubing of different diameters help secure tighter connections and avoid leakage in the system (G). Figures 2B, D and F were created with BioRender. Scale bars = 2 cm. Please click here to view a larger version of this figure.
3. Sample preparation and mounting
- Wash the sample by submerging it in water for 2 min.
CAUTION: Do not wash samples in the sink, as all contrasting solutions include heavy metal salts in their composition. Consider the water used for washing as a diluted contrasting solution and dispose of it appropriately. - Place the sample in a paper towel at room temperature to allow excess water to evaporate for 2-5 min depending on the size of the sample. Alternatively, dry the sample slightly with the aid of a paper towel. Do not allow the sample to dry out completely.
- Wrap the sample in a paraffin film by stretching it to a thin layer. Avoid folding the paraffin film on top of the sample.
- Mount the wrapped sample onto a sample holder, keeping it stable and in position while it rotates during the scanning. Use adhesive tape, low-density foam, pipette tips, and/or clear plastic containers to secure the sample in place.
- Scan the sample and analyze the images following specific protocols and guidelines established for the micro-CT system available.
Representative Results
The haustorium of parasitic plants is a complex organ comprising different tissues and cell types that intertwine and connect with the tissues of another plant, used as a host20. Micro-CT scanning can be leveraged to better understand this complex structure in a non-destructive and three-dimensional way when analyzing both small (Figure 1A-C) and large (Figure 1D,E) haustoria. To do this, contrasting solutions can be applied into the parasite-host interface, thus making it possible to analyze samples that would otherwise have low and homogenous X-rays absorption. The two simple approaches described in this protocol rely on pressurizing the contrasting solution to accelerate penetration through the sample. As expected, the protocols described here work for different micro-CT systems. However, scanning settings and parameters differ depending on the system and the analyzed sample (Table 1).
| Functional group | Euphytoid parasite | Endoparasite | Parasitic vine | Mistletoe (before/after contrast) | Obligate root parasite |
| Species (Family) | Pyrularia pubera | Viscum minimum | Cuscuta americana | Struthanthus martianus | Scybalium fungiforme |
| Family | Santalaceae | Viscaceae | Convolvulaceae | Loranthaceae | Balanophoraceae |
| Contrast solution | 3% phosphotungstate | 1% iodine | 1% iodine | 1% iodine | 0.2% lead nitrate |
| Application method | vacuum | vacuum | perfusion | perfusion | perfusion |
| Micro-CT system | Zeiss Versa 620 | Nikon X-Tek HMXST225 | Bruker Skyscan 1176 | Bruker Skyscan 1176 | Bruker Skyscan 1176 |
| Projections | 4500 | 3140 | 610 | 1200 / 2400 | 5300 |
| Frames | 1 | 1 | 3 (averaged) | 3 / 3 (averaged) | 3 (averaged) |
| Exposure (ms) | 5000 | 1000 | 680 | 1600 / 830 | 750 |
| Filter (mm) | none | Al 0.25 | Al 1.0 | Al 1.0 / Al 1.0 | none |
| Voltage (kV) | 60 | 85 | 35 | 90 / 45 | 40 |
| Current (μA) | 108 | 80 | 375 | 180 / 390 | 600 |
Table 1: Scanning settings and parameters for the analyzed samples.
Following the first approach for contrast application (step 2.3), vacuum was used to perfuse the haustorium of the euphytoid parasite P. pubera with a 3% phosphotungstate solution for two hours. The sample was then scanned in a 3D X-ray microscope (XRM) system (see Table of Materials), achieving high image resolution via secondary optical magnification4. XRM images were observed to be as effective as anatomical sections analyzed under a light microscope to analyze tissue organization and topology at the parasite-host interface (Figure 3). The use of phosphotungstate highlights the abundance of parenchyma tissue, which appears in a bright white tone due to the high absorption of the contrasting agent. Vessels appear in a dark gray tone due to low absorption of contrast. Based on this color difference, it is possible to observe the abundance of parenchyma surrounding the vascular core of the haustorium (Figure 3). The intricate vessels and thin parenchyma strands of the vascular core itself are also observed, especially in longitudinal sections (Figures 3A-C). In cross-sections, the vascular core is easily observed as two vascular strands separated by parenchyma (Figures 3D,E).
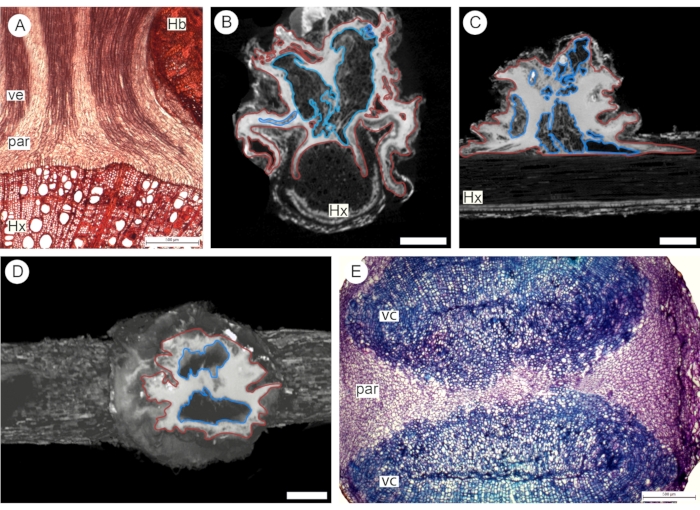
Figure 3: Euphytoid parasite haustorium. Longitudinal (A-C) and transversal (D-E) sections through the haustorium were observed under a light microscope (A,E) and via 3D X-ray microscopy following application of 3% phosphotungstate solution using vacuum (B-D). Red outlines indicate parenchyma tissue (par), and blue outlines indicate the vascular core (vc). Hx: host xylem. Hb: host bark. Scale bars = 500 µm (A, E) and 2.5 cm (B-D; voxel size = 2.8382 µm). Please click here to view a larger version of this figure.
The same approach was used to introduce contrast into the stem of a succulent host plant (Euphorbia polygona, Cactaceae) parasitized by V. minimum. Here, 1% iodine solution was chosen as a contrasting agent based on preliminary histochemical analysis, which showed that parenchyma cells of the endoparasite store carbohydrates in the form of starch (Figure 4A). At the same time, cells in the host cortex do not store a detectable amount of starch (Figure 4B-D). After contrast application, the sample was then scanned using a Nikon X-Tek micro-CT system (see Table of Materials). The difference in iodine absorption allowed the detection of the intricate web of cortical strands formed by the endoparasite within the host body (Figure 4E). Following this methodology, cortical strands were observed to concentrate around the host vascular center and eventually branch towards the periphery of the host stem when associated with the emersion of a parasite flower (Figures 4F,G).
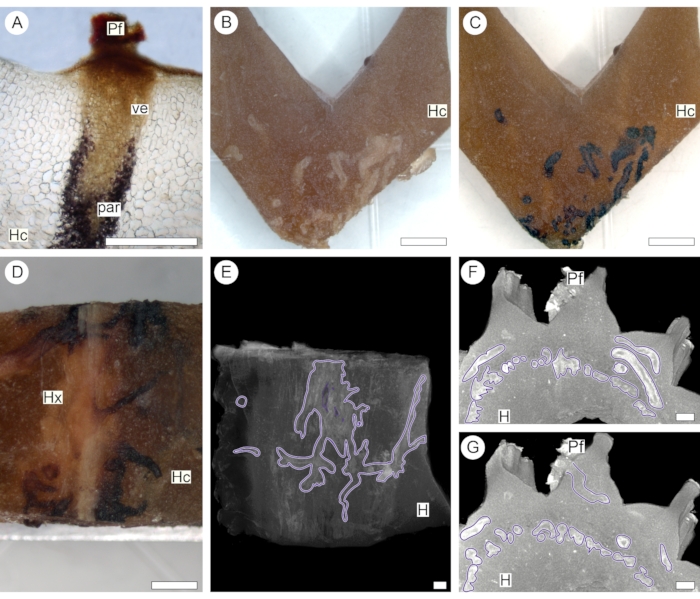
Figure 4: Endoparasite tissue network. Anatomical (A) and macro (B-D) sections show reaction with 1% iodine solution indicating that starch (stained in black) is present in the parenchyma (par) associated with the vessels (ve) of the parasite. Because starch is absent in the host cortex (Hc) and xylem (Hx), 1% iodine solutions were selectively used to enhance the contrast of parasite cortical strand tissues (purple outlines, E-G), seen against the host background (H). The presence of a flower (Pf) confirms that the stained tissue is part of the endoparasite structure (A,F,G). Scale bars = 0.25 cm. Please click here to view a larger version of this figure.
In the second approach described here (step 2.4), a supply tank containing the contrasting solution is elevated from the level in which the sample is placed, thus using gravity to drive the passage of the solution through the sample. After contrast perfusion, scanning was carried out using a Bruker Skycan micro-CT system (see Table of Materials). Results obtained for C. americana (Figures 5A,B) and S. martianus (Figures 5C-G) help illustrate the convenience of this approach for both small and large samples, respectively. Comparing samples scanned before (Figure 5C,E) and after (Figure 5D,F) perfusion of 1% iodine solution stresses the importance of contrast application even in woody haustorium samples. It is noteworthy that in the associations between both C. americana and S. martianus (Figure 5) and their respective hosts, parasites have little to no starch content in their tissues. This explains the different results described for the endoparasite V. minimum (Figure 4), in which the same method and type of contrast solution were used.
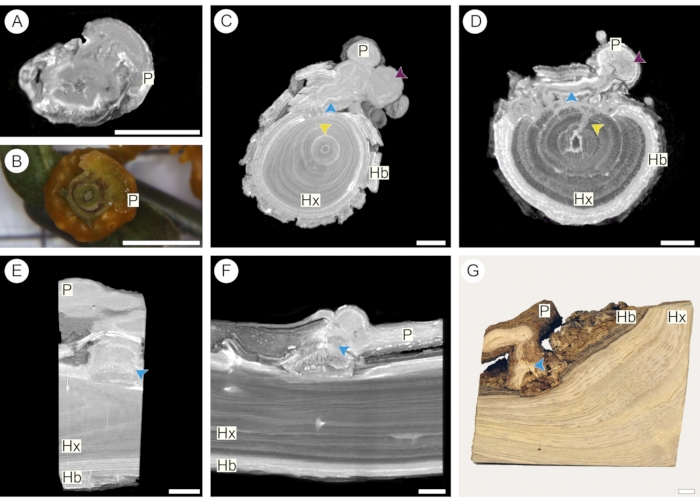
Figure 5: Parasitic vine and mistletoe haustorium. Longitudinal sections through the haustorium of a parasitic vine (A,B) and a mistletoe (C-G). Comparison between scan and macro section of fresh material shows that the intricate haustorium structure is well captured in micro-CT scans (A,B). Comparison between fixated samples before (C,E) and after (D,F) the perfusion of 1% iodine solution show that contrast is enhanced even in lignified samples, facilitating the observation of parasites (P) structures such as vessels in the epicortical root (pink arrowheads), vascular strands (blue arrowheads), and sinker (yellow arrowhead). Vessels and annual rings of the host xylem (Hx) and starch in the host bark (Hb) are also more easily observed. Scale bars = 1 cm. Please click here to view a larger version of this figure.
A final result obtained with the perfusion protocol described here is the possibility to detect vascular connections between parasites and host plants. This was achieved by perfusing a 0.2% lead nitrate solution through a segment of the host root around which the tuber of the obligate root parasite S. fungiforme had developed. After contrast application, scanning was also carried out using a Bruker Skyscan micro-CT system (see Table of Materials). Sequential projections show that vessels in the host root bifurcate into the parasite tuber (Figure 6A-C). Following the analysis of these results, the same sample was cut into a smaller sub-sample, prepared for anatomical study, and analyzed using light microscopy. Serial sectioning of this sub-sample confirms that the xylem continuity between the two plants is formed by vessel-to-vessel connection via perforation plates (Figure 6D-F).
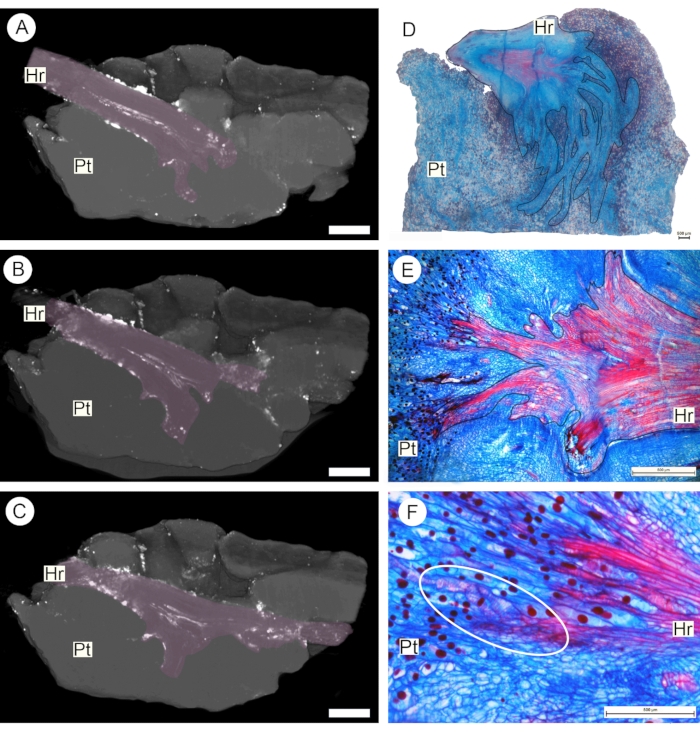
Figure 6: Obligate root parasite haustorium. Longitudinal sections through the haustorium as observed via micro-CT scanning (A-C) and anatomical sections observed under the light microscope (D-F). The accumulation of 0.2% lead nitrate solution within the vascular system of the host root (Hr, pink outline) allows observation of host vessels branching within the parasite tube (Pt) and the detection of direct vascular connection between the two plants (dashed white outline). Scale bars = 1 cm (A-C) and 500 µm (D-F). Please click here to view a larger version of this figure.
Discussion
The use of heavy metal solutions to improve plant tissue contrast has become a crucial step in sample preparation for micro-CT analysis. Several compounds commonly available in plant micro-morphology laboratories have been tested by Staedler et al., who recommend using phosphotungstate as the most effective agent in penetrating samples and increasing contrast index8. Results obtained here in the analysis of the haustorium of P. pubera corroborate this recommendation. In terms of contrast application, Steadler et al. describe that the contrast solution was passively infiltrated through the analyzed material (flowers and flower buds ranging from 1 mm to 10 mm) during 1 to 8 days8. Other authors have used the same protocol of passive infiltration over extended periods (1-4 weeks) when analyzing larger samples, such as the 30 cm wide flowers of endoparasitic Rafflesiaceae species28. While this process has proven successful, results obtained with the protocol described here show that the infiltration process can be significantly accelerated under vacuum, thus improving the previously established method. A vacuum chamber or pump forces air to escape from the plant tissues, thus allowing easier infiltration of the contrast solution. Vacuum can be applied even to fragile structures, as shown here for the haustorium of P. pubera and the soft tissues of the succulent host plant E. polygona parasitized by V. minimum. Moreover, the use of vacuum pumps is a common procedure for the infiltration of fixatives or embedding substances in many laboratories of plant micro-morphology, thus making this protocol more accessible.
The same approach was used here with a contrasting agent that selectively stains samples if specific storage compounds are present. When applied through vacuum or passive infiltration, selective staining might provide poorer contrast enhancement to an entire sample when compared to phosphotungstate, for instance. On the other hand, it is beneficial for highlighting specific plant structures or tissues. As reported here, when combined with previous anatomical or histochemical analysis, the use of selective contrast agents such as iodine can aid in the differentiation of parasitic plant tissue within the host's body provided that either parasite or host (but not both) show abundant starch reserves. Selectively increasing X-ray absorption of parasite tissues is shown to be especially useful for exploring, for the first time, the complex three-dimensional network formed by an endoparasitic plant such as V. minimum within the stem of its host plant. Moreover, it is noteworthy that endoparasitic plants and certain fungi show an interesting evolutionary convergence, both growing "incognito" inside their hosts for much of their life cycles, emerging only during brief periods29. Thus, a potential new application of the protocol described here would be to use phloxine B, a stain containing bromine30, to detect endophytic fungi within plant tissues. Eosin, another bromine-based staining solution only seldomly used for plant tissues, has been shown to enhance contrast in mouse kidney samples analyzed using a specific micro-CT system31.
Another approach described in this protocol is the perfusion of contrasting agents through the vascular tissue of the host stem or root. This approach, which differs significantly from previously reported methods, is based on the work of Sperry et al., who perfused wood samples with safranin dye to analyze hydraulic conductivity32. As discussed by the authors and highlighted here, a critical step in the protocol is setting up the stain-perfusing apparatus to avoid introducing air bubbles into the system32. Three-way valves can temporarily diverge the solution flux and eliminate bubbles from the liquid comlumn32. Nevertheless, emboli can be present inside the vessels of the host plant, caused either artificially during sampling or naturally due to the generally high transpiration rates of parasitic plants33,34. This can severely decrease perfusion efficiency, leading to contrast not being improved in the sample. It is essential to apply low to moderate vacuum when fixating the specimen after sampling to prevent this issue. Complementarily, the fixated specimen can be flushed under high pressure using the fixative solution in which the sample is stored. Flushing can help restore conductivity in the sample by removing air bubbles32, thus clearing the path for perfusion of the contrast solution. If working with fresh material, introduction of air bubbles into the plant can be avoided by submerging the specimen in water immediately after sampling, then cutting it again underwater, and smoothing the end surface with sharp blades35.
Following this approach, 1% iodine solution was perfused through the haustorium of C. americana and S. martianus to enhance the contrast of the host-parasite interface as a whole. Results obtained for these species show that the perfusion protocol described here works well for both fresh and fixated samples, and that introduction of contrast solutions improves visualization even in lignified samples. These observations agree with results reported by Teixeira-Costa & Ceccantinti17, who used the same approach to apply a 0.2% lead nitrate solution to visualize specific aspects of haustorium structure. In contact with carbon dioxide, lead nitrate reacts to form a highly insoluble precipitate composed of lead carbonate crystals, which efficiently absorb X-rays8,36. Once perfused in the sample vascular tissue, this precipitate clogs up vascular pit connections, allowing the solution to flow only through direct, vessel-to-vessel connections via perforation plates. Following this approach, the presence of direct parasite-host xylem connections through perforation plates is shown here for S. fungiforme and confirmed with detailed anatomical analysis. These results highlight a further application of this approach to test haustorium functionality. Given that haustorium initiation might not culminate in the development of a fully functional organ due to either parasite-host incompatibility or host resistance against the parasite, the approach described here can be used to test whether vascular connections are formed between the two plants leading to the occurrence of effective parasitism. Considering the value of using micro-CT scanning to investigate xylem embolisms more broadly12,37, the protocol presented here can also improve the visualization and quantitative analysis of xylem embolism in other non-parasitic plants species.
In conclusion, this protocol approaches one of the critical advances expected in plant microtomography, which is applying contrasting agents to help differentiate structures with low X-ray absorpion24. As shown here, contrast solutions can significantly improve the visualization of haustorium structures at the interface between parasitic plants and their hosts. Different compounds can be chosen according to their specificity in reacting with reserve compounds, such as starch, which is often differently distributed among parasite and host tissues. The approach to contrast application into haustorium samples can also be chosen to investigate specific features of the parasite-host interface, such as direct vessel-to-vessel connection. Furthermore, this protocol may also be applied to non-parasitic species, potentially improving the detection of endophytic fungi and quantification of xylem embolism. In any application, the protocol described here to improve microtomography analysis can be combined with other tools, such as optical projection tomography and automated virtual segmentation, to provide new and exciting insights into the three-dimensional development of the connection between these plants and their hosts38,39.
Declarações
The authors have nothing to disclose.
Acknowledgements
I would like to thank Dr. Simone Gomes Ferreira (Microtomography Laboratory, University of Sao Paulo, Brazil) and Dr. Greg Lin (Center for Nanoscale Systems, Harvard University, USA) for their paramount help and indispensable user training for different microtomography systems and data analysis software. I also thank the staff at the EEB Greenhouse at the University of Connecticut (USA), especially Clinton Morse and Matthew Opel for providing the specimens of Viscum minimum. Dr. John Wenzel provided the opportunity and great help for the sampling of Pyrularia pubera. MSc. Carolina Bastos, MSc. Yasmin Hirao, and Talitha Motta greatly helped with the sampling of Scybalium fungiforme. MSc. Ariadne Furtado, and Drs. Fernanda Oliveira and Maria Aline Neves provided the reference for the use of phloxine B for the analysis of endophytic fungi. Video recording at the Vrije Universiteit Brussel was made possible through the help of Dr. Philippe Claeys, Dr. Christophe Snoeck, MSc. Jake Griffith, Dr. Barabara Veselka, and Dr. Harry Olde Venterink. Funding was provided by the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) and the Harvard University Herbaria (USA).
Materials
| 3D X-ray microscope (XRM) system | Zeiss Versa 620 | used to scan Pyrularia pubera | |
| 3D X-ray microscope + A2:D22 | Zeiss | Versa 620 | Used for scanning the species P. pubera |
| CT-Pro 3D software | Nikon | version XT 3.1.11 | Used for three-dimensional reconstruction of scans |
| CT-Vox software | Bruker | version 3.3.1 | Used for analyses and acquisition of images and videos |
| Dragonfly software | Object Research Systems – ORS | version | Used for analyses and acquisition of images and videos |
| Glass vials | Glass Vials Inc. SE | V2708C-FM-SP | Sold by VWR – USA; make sure that vials are able to withstand vacuum at ca. 10 psi |
| Inspect-X | Zeiss | version XT 3.1.11 | Used for controlling the Nikon X-Tek HMXST225 system |
| Iodine solution 0.0282 N | WR Chemicals BDH | BDH7422-1 | Sold by VWR – USA |
| Lead Nitrate II PA 500 g | Vetec | 361.08 | Sold by SPLab |
| Microtomography scanner | Bruker | Skyscan1176 | Used for scanning the species C. americana, S. martianus, and S. fungiforme |
| Microtomography scanner | Nikon | X-Tek HMXST225 | Used for scanning the species V. minimum |
| NRecon software | Bruker | version 1.0.0 | Used for three-dimensional reconstruction |
| Phosphotungstic acid hydrate 3% in aqueous solution | Electron Microscopy Sciences | 101410-756 | Sold by VWR – USA |
| Plastic film (Parafilm) | Heathrow Scientific | PM996 | Sold by VWR – USA |
| Plastic IV bag 500 mL | Taylor | 3478 | Sold by Fibra Cirurgica Produtos para Saude |
| PVC tubing 3/4'' | Nalge Nunc International | SC63013-164 | Sold by VWR – USA |
| Scanning system | Nikon X-Tek HMXST225 | used to scan Viscum minimum | |
| Scanning system | Bruker Skyscan 1176 | used to scan C. americana | |
| Scout-and-ScanTM software | Zeiss | version 16 | Used for controlling the Zeiss Versa 620 system and for three-dimensional reconstruction of scans |
| Three-way valve | ToToT | DMTWVS-5 | Sold by Amazon USA |
| Two-part syringe | HSW Henke-Ject | 4850001000 | Used without the plunger |
| Vacuum chamber | Binder | 80080-434 | Sold by VWR – USA; includes pump and connecting tubes |
| VG Studio Max software | Volume Graphics | version 3.0 | Used for analyses and acquisition of images and videos |
Referências
- Stock, S. R. . Microcomputed tomography: Methodology and applications. , (2020).
- Hounsfield, G. N. Computerized transverse axial scanning (tomography): I. Description of system. British Journal of Radiology. 46 (552), 1016-1022 (1973).
- Dutilleul, P., Lafond, J. A. Editorial: Branching and rooting out with a CT Scanner: The why, the how, and the outcomes, present and possibly future pierre. Frontiers in Plant Science. 7 (41), 5-6 (2016).
- Metscher, B. D. Biological applications of X-ray microtomography: Imaging micro- anatomy, molecular expression and organismal diversity. Microscopy and Analysis. 27 (2), 13-16 (2013).
- Sakdinawat, A., Attwood, D. Nanoscale X-ray imaging. Nature Photonics. 4 (12), 840-848 (2010).
- Walton, L. A., et al. Morphological characterisation of unstained and intact tissue micro-architecture by X-ray computed micro- and nano-tomography. Scientific Reports. 5, 1-14 (2015).
- Lafond, J. A., Han, L., Dutilleul, P. Concepts and analyses in the ct scanning of root systems and leaf canopies: A timely summary. Frontiers in Plant Science. 6 (1111), 85-91 (2015).
- Staedler, Y. M., Masson, D., Schönenberger, J. Plant tissues in 3D via X-Ray Tomography: Simple contrasting methods allow high resolution imaging. PLoS ONE. 8 (9), 75295 (2013).
- Heeraman, D. A., Hopmans, J. W., Clausnitzer, V. Three dimensional imaging of plant roots in situ with X-ray Computed Tomography. Plant and Soil. 189, 167-179 (1997).
- Dhondt, S., Vanhaeren, H., Van Loo, D., Cnudde, V., Inzé, D. Plant structure visualization by high-resolution X-ray computed tomography. Trends in Plant Science. 15 (8), 419-422 (2010).
- McElrone, A. J., Choat, B., Parkinson, D. Y., MacDowell, A. A., Brodersen, C. R. Using high resolution computed tomography to visualize the three dimensional structure and function of plant vasculature. Journal of Visualized Experiments. (74), e50162 (2013).
- Cochard, H., Delzon, S., Badel, E. X-ray microtomography (micro-CT): A reference technology for high-resolution quantification of xylem embolism in trees. Plant, Cell and Environment. 38 (1), 201-206 (2015).
- Bastos, C. L., Tamaio, N., Angyalossy, V. Unravelling roots of lianas: A case study in Sapindaceae. Annals of Botany. 118 (4), 733-746 (2016).
- da Cunha Neto, I. L., et al. Diversity, distribution, development, and evolution of medullary bundles in Nyctaginaceae. American Journal of Botany. 107 (5), 707-725 (2020).
- Milien, M., Renault-Spilmont, A. S., Cookson, S. J., Sarrazin, A., Verdeil, J. L. Visualization of the 3D structure of the graft union of grapevine using X-ray tomography. Scientia Horticulturae. 144, 130-140 (2012).
- Paya, A. M., Silverberg, J. L., Padgett, J., Bauerle, T. L. X-ray computed tomography uncovers root-root interactions: Quantifying spatial relationships between interacting root systems in three dimensions. Frontiers in Plant Science. 6 (274), 54-65 (2015).
- Teixeira-Costa, L., Ceccantini, G. C. T. Aligning microtomography analysis with traditional anatomy for a 3D understanding of the host-parasite interface – Phoradendron spp. Case study. Frontiers in Plant Science. 7, 1340 (2016).
- Lusic, H., Grinstaff, M. W. X-ray-computed tomography contrast agents. Chemical Reviews. 113 (3), 1641-1666 (2013).
- Těšitel, J. Functional biology of parasitic plants: a review. Plant Ecology and Evolution. 149 (1), 5-20 (2016).
- Teixeira-Costa, L. A living bridge between two enemies: Haustorium structure and evolution across parasitic flowering plants. Revista Brasileira de Botanica. 44 (1), 165-178 (2021).
- Kuijt, J. . The Biology of Parasitic Flowering Plants. , (1969).
- Masumoto, N., et al. Three-dimensional reconstructions of haustoria in two parasitic plant species in the Orobanchaceae. Plant Physiology. 185 (4), 1429-1442 (2021).
- Calo, C. M., et al. A correlation analysis of Light Microscopy and X-ray MicroCT imaging methods applied to archaeological plant remains’ morphological attributes visualization. Scientific Reports. 10 (1), 1-15 (2020).
- Brodersen, C. R., Roddy, A. B. New frontiers in the three-dimensional visualization of plant structure and function. American Journal of Botany. 103 (2), 184-188 (2016).
- Teixeira-Costa, L., Davis, C. C. Life history, diversity, and distribution in parasitic flowering plants. Plant Physiology. 187 (1), 32-51 (2021).
- Simpson, B. B. Krameriaceae. Flora Neotropica Monograph. 49, (1989).
- Ruzin, S. E. . Plant microtechnique and microscopy. , (1999).
- Nikolov, L. A., Tomlinson, P. B., Manickam, S., Endress, P. K., Kramer, E. M., Davis, C. C. Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Annals of Botany. 114, 233-242 (2014).
- Thorogood, C. J., Teixeira-Costa, L., Ceccantini, G., Davis, C., Hiscock, S. J. Endoparasitic plants and fungi show evolutionary convergence across phylogenetic divisions. New Phytologist. 232 (3), 1159-1167 (2021).
- Largent, D., Johnson, D., Watling, R. . How to Identify Mushrooms to Genus III: Microscopic Features. , (1977).
- Busse, M., et al. Three-dimensional virtual histology enabled through cytoplasm-specific X-ray stain for microscopic and nanoscopic computed tomography. Proceedings of the National Academy of Sciences of the United States of America. 115 (10), 2293-2298 (2018).
- Sperry, J. S., Donnelly, J. R., Tyree, M. T. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment. 11, 35-40 (1988).
- Calvin, C. L. Host-formed tyloses in vessels of the mistletoe Phoradendron (Viscaceae). IAWA Journal. 18 (2), 117-126 (1997).
- Teixeira-Costa, L., Ceccantini, G. Embolism increase and anatomical modifications caused by a parasitic plant. IAWA Journal. 36 (2), 138-151 (2015).
- Ellmore, G. S., Ewers, F. W. Fluid flow in the outermost xylem increment of a ring-porous tree, Ulmus americana. American Journal of Botany. 73 (12), 1771-1774 (1986).
- Ellis, E. A. Staining sectioned biological specimens for transmission electron microscopy: Conventional and En bloc stains. Electron Microscopy: Methods and Protocols. 1117, 57-72 (2014).
- Brodersen, C. R., McElrone, A. J., Choat, B., Matthews, M. A., Shackel, K. A. The dynamics of embolism repair in xylem: In vivo visualizations using high-resolution computed tomography. Plant Physiology. 154 (3), 1088-1095 (2010).
- Brodersen, C. R., et al. Automated analysis of three-dimensional xylem networks using high-resolution computed tomography. New Phytologist. 191 (4), 1168-1179 (2011).
- Lee, K., et al. Visualizing plant development and gene expression in three dimensions using optical projection tomography. Plant Cell. 18 (9), 2145-2156 (2006).

