Microwave-Assisted Preparation of 1-Aryl-1H-pyrazole-5-amines
Summary
1-Aryl-1H-pyrazole-5-amines are prepared from aryl hydrazines combined with either 3-aminocrotononitrile or an α-cyanoketone in a 1 M HCl solution using a microwave reactor. Most reactions are done in 10-15 minutes and pure product can be obtained via vacuum filtration with typical isolated yields of 70-90%.
Abstract
A synthetic process for the preparation of a variety of 1-aryl-1H-pyrazole-5-amines was developed. The microwave-mediated nature of this method makes it efficient in both time and resources and utilizes water as the solvent. 3-Aminocrotononitrile or an appropriate α-cyanoketone is combined with an aryl hydrazine and dissolved in 1 M HCl. The mixture is then heated in a microwave reactor at 150 °C, typically for 10-15 min. The product can be readily obtained by basifying the solution with 10% NaOH and isolating the desired compound with a simple vacuum filtration. The use of water as a solvent in this reaction lends to its ease and utility in production, and this method is easily reproducible with a variety of functional groups. Typical isolated yields range from 70-90%, and reactions can be performed on the milligram to gram scale with little to no change in observed yields. Some of the applications of these molecules and their derivatives include pesticides, anti-malarials, and chemotherapeutics, among many others.
Introduction
The impetus to create a streamlined synthesis of 1-aryl-1H-pyrazole-5-amines is due to the myriad of applications of these small molecules. They appear in kinase inhibitors1, antibiotics2, pesticides3, and among many other biologically active compounds4,5. Synthetic schemes for these compounds are abundant, but most involve complex isolation and purification techniques. A common method involves the reflux of aryl hydrazine and 3-aminocrotononitrile in either alcoholic or aqueous solutions followed by a subsequent purification via chromatography and/or recrystallization6,7,8,9,10. A handful of isolated reports have detailed the synthesis of these compounds using microwave radiation, but all required extensive heating time and offered little advantage when compared to other previously reported methods11,12.
Despite their utility, there are a limited number of 1-aryl-1H-pyrazole-5-amines available from commercial vendors. We recently had success preparing nitrogen heterocycles using a microwave reactor13 and decided to investigate a related methodology for pyrazole-5-amine analogs. In this paper, we detail our procedure to prepare 1-aryl-1H-pyrazole-5-amines by reacting an aryl hydrazine with either 3-aminocrotononitrile or an α-cyanoketone in 1 M HCl under microwave radiation. The advantages of this procedure include a short reaction time and the ability to incorporate a variety of functional groups including halides, nitriles, phenols, sulfones and nitro groups14.
Protocol
CAUTION: Please review all relevant material safety data sheets (MSDS) before use. Follow all appropriate safety practices when using the microwave reactor including reviewing the microwave reactor protocols and the use of personal protective equipment (safety glasses, gloves, lab coat, full-length pants, closed-toe shoes). This procedure is designed to work using an aryl hydrazine and either 3-aminocrotononitrile or an α-cyanoketone. The appropriate microwave vial and stir bar should be used according to the scale of the reaction as specified by the manufacturer.
1. Preparation of reaction mixture
NOTE: The following reaction between 4-fluorophenylhydrazine hydrochloride and 3-aminocrotononitrile on a 2 mmol scale is representative. The procedure is identical when substituting α-cyanoketones in place of 3-aminocrotononitrile14.
- Obtain a microwave vial designed for reaction volumes of 2-5 mL that has been dried overnight in a glassware oven and add an appropriate stir bar.
- Add 0.325 g of 4-fluorophenylhydrazine hydrochloride (1 equiv., 2 mmol) and 0.164 g of 3-aminocrotononitrile (1 equiv., 2 mmol) to the microwave vial.
- Add 5 mL of 1 M HCl to make the concentration of starting reagents as 0.4 M. Using a stir plate, ensure that the heterogeneous suspension is stirred. Add additional solvent if reactants have poor solubility and the reaction mixture cannot be stirred properly. Transfer the solution to a larger vial if necessary, to avoid exceeding the recommend solvent volume as specified by the microwave reactor operating manual.
2. Heating the reaction in the microwave reactor
- Seal the microwave vial with a microwave vial cap using the appropriate crimper tool.
- Place the vial in the microwave reactor. Program the microwave settings for time (10 min), temperature (150 °C), and absorption (Very High).
CAUTION: Pay attention to the reactor pressure during the heating phase. A sudden drop in pressure may indicate a leak and/or vessel failure. - Once the reaction has cooled (< 40 °C), remove the vial from the microwave reactor.
- Remove the cap using an appropriate decapper tool.
3. Isolation of the product by vacuum filtration
- In a well ventilated fume hood, clamp the microwave vial over a stir plate.
- Add 2 mL of 10% NaOH with stirring to make the solution alkaline and cause immediate precipitation of product. The pH of the solution should be >10 as indicated by pH paper. Sonication and scraping the vial with a spatula can aid with mixing and dislodging product from the vessel walls.
NOTE: Some products oil out of the alkaline solution. If this occurs, transfer the solution with the aid of ~20 mL of deionized water to a separatory funnel and extract 3x with dichloromethane or ethyl acetate. Dry the combined organic layers and evaporate to obtain the product. - Set up a vacuum filtration apparatus to isolate solid product precipitate. Use deionized water to rinse any remaining product from the microwave vial and wash the isolated product.
- Allow the product to dry overnight on the bench top or in a desiccator. Isolated yields are typically in the 70-90% range14.
- Obtain a 1H NMR spectrum in CDCl3 to confirm product identity and purity.
Representative Results
In this demonstration, 3-aminocrotononitrile and 4-fluorophenylhydrazine hydrochloride were reacted to produce 1-(4-fluorophenyl)-3-methyl-1H-pyrazol-5-amine (Figure 1). The mixture of the starting material in the microwave vial seen in Figure 2a shows the heterogeneous suspension created by combining the starting materials in the 1 M HCl solvent. It is recommended to pre-mix the solution for a few seconds over a stir plate to ensure that the stir bar can freely spin. After the microwave reaction occurs, the solution becomes a homogenous mixture as seen in Figure 2b. After stirring and adding enough 10% NaOH to make the solution basic, the product will rapidly precipitate out of the solution as shown in Figure 2c. The precipitate can then be filtered and washed with deionized water producing the solid seen in Figure 2d after drying. If the product oils out of solution (Figure 2e), it can instead be isolated by extracting with either dichloromethane or ethyl acetate. Once the product is isolated and dried, it can be characterized via NMR to confirm the synthesis of the target 5-aminopyrazole (Figure 3).
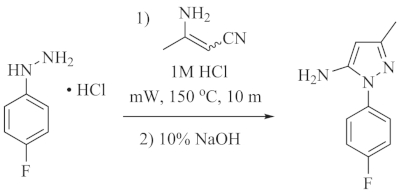
Figure 1. Synthesis of 1–(4–fluorophenyl)–3–methyl–1H–pyrazol–5–amine. Reaction scheme showing the reaction between 3-aminocrotononitrile and 4-fluorophenylhydrazine hydrochloride to obtain the desired product. Please click here to view a larger version of this figure.
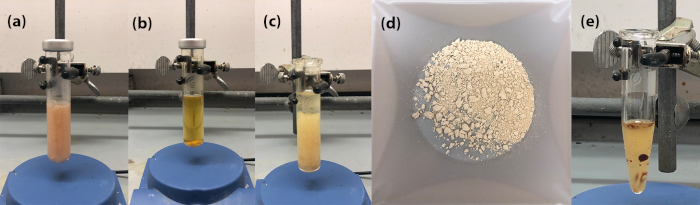
Figure 2. Images of the solution during the course of the reaction. (a) Reaction mixture prior to heating. (b) Reaction mixture after heating in the microwave. (c) Precipitation of product after addition of enough 10% NaOH to make the solution alkaline (pH > 10). (d) Appearance of the product after isolation by vacuum filtration. (e) Example of a product (3-methyl-1-(3-methylphenyl)-1H-pyrazol-5-amine) that oils out of solution after addition of 10% NaOH, requiring an extraction to isolate. Please click here to view a larger version of this figure.
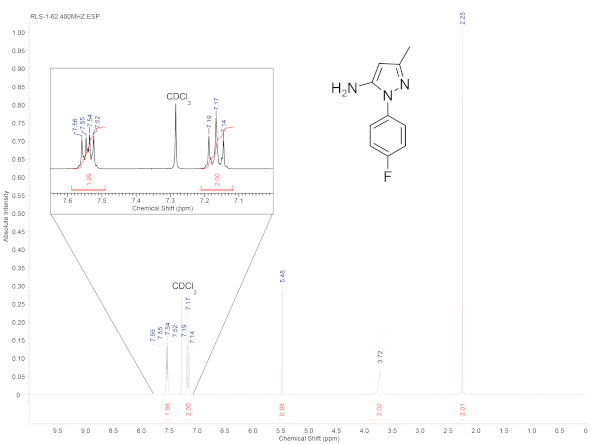
Figure 3. 1H NMR (400 MHz) spectrum of 1–(4–fluorophenyl)–3–methyl–1H–pyrazol–5–amine in CDCl3. Spectrum demonstrating the purity of the product isolated by a simple filtration. Please click here to view a larger version of this figure.
Discussion
A number of 1-aryl-1H-pyrazole-5-amines were prepared by combining an α-cyanoketone or 3-aminocrotonitile with an aryl hydrazine in 1 M HCl and heating the solution to 150 °C in a microwave reactor. Nearly all compounds were synthesized in 10-15 min, with the slowest substrate requiring 35 min of heating14. The use of water as the solvent allows for rapid heating of the solution and minimizes the use of hazardous organic solvents.
Higher temperature and pressures can certainly be achieved in microwave reactors, but the reaction mixture was only heated to 150 °C in order to keep operating pressures below 10 bar as a matter of safety. Higher temperatures would be expected to reduce reaction times and many commercial microwave reactors can handle higher pressures. However, our experience is that reactor vessel failure becomes much more frequent at pressures above 15 bar and some organic reactants decompose at higher temperatures.
Some substrates showed limited solubility in 1 M HCl, but solubilize as the reaction progresses (Figure 2). However, if the substrates do not dissolve to any appreciable extent in the reactor vessel, this can cause the stir bar to become stuck and lead to an unsuccessful reaction. As with all microwave reactions, proper stirring is critical to reaction success, as most waveguides direct the radiation to the top of the solution, making a stirred solution critical to distributing the heat and maintaining a uniform heat distribution profile. In these cases, more solvent can be used to ensure proper stirring of the solution. While higher concentrations of HCl can also be used, we saw no appreciable difference in either reaction times or the solubility of reactants. Lower concentrations of HCl, however, led to thick suspensions which could not be stirred efficiently. Once the reaction is basified, the majority of compounds readily precipitate from solution and are easily captured via vacuum filtration. Some compounds, however, may oil out in solution. In these cases, a liquid-liquid extraction with either ethyl acetate or dichloromethane is required to isolate the product.
In conclusion, this methodology allows for the rapid preparation of 1-aryl-1H-pyrazole-5-amines without the need for extensive purification protocols found in other reported procedure. The ease of the procedure and workup allows most products to be isolated in less than 1 h, and the use of water as the solvent helps to improve safety and reduce environmental impact and disposal cost. This procedure tolerates a wide range of functional groups, and we expect further production of novel compounds utilizing this methodology.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Bill and Linda Frost Fund.
Materials
| 2-5mL Microwave vial set | Chemglass | CG-4920-01 | Set includes appropriate stir bars and 20mm aluminum seals |
| Biotage Initiator+ microwave | Biotage | 356007 | Includes crimper and decapper tool. |
| Sonicator | Kendal | Ultrasonic Cleaner GB-928 | |
| Glassware oven | Quincy Lab | 20GC | |
| 4-Fluorophenylhydrazine hydrochloride | Fisher | AC119590100 | |
| 3-Aminocrotonitrile | Fisher | AC152451000 | |
| CDCl3 | Cambridge Labs | DLM-7-100 | 99.8% D |
| Hydrochloric acid, concentrated | Fisher | A144SI-212 | Used to prepare 1 M HCl solution |
| Sodium hydroxide pellets | Fisher | S318-100 | Used to prepare 10% NaOH solution |
Referências
- Gradler, U., et al. Fragment-based discovery of focal adhesion kinase inhibitors. Bioorganic & Medicinal Chemistry Letters. 23 (19), 5401-5409 (2013).
- Chandak, N., Kumar, S., Kumar, P., Sharma, C., Aneja, K. R., Sharma, P. K. Exploration of antimicrobial potential of pyrazolo[3,4-b]pyridine scaffold bearing benzenesulfonamide and trifluoromethyl moieties. Medicinal Chemistry Research. 22 (11), 5490-5503 (2013).
- Huo, J., et al. Synthesis and biological activity of novel N-(3-furan-2-yl-1-phenyl-1H-pyrazol-5-yl) amides derivatives. Chinese Chemical Letters. 27 (9), 1547-1550 (2016).
- Anand, D., et al. Antileishmanial activity of pyrazolopyridine derivatives and their potential as an adjunct therapy with miltefosine. Journal of Medicinal Chemistry. 60 (3), 1041-1059 (2017).
- Eldehna, W. M., El-Naggar, D. H., Hamed, A. R., Ibrahim, H. S., Ghabbour, H. A., Abdel-Aziz, H. A. One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDA-MB-231 cells. Journal of Enzyme Inhibition and Medicinal Chemistry. 33 (1), 309-318 (2017).
- Briebenow, N., et al. Identification and optimization of substituted 5-aminopyrazoles as potent and selective adenosine A1 receptor antagonists. Bioorganic & Medicinal Chemistry Letters. 20 (19), 5891-5894 (2010).
- Marinozzi, M., et al. Pyrazole[3,4-e][1,4]thiazepin-7-one derivatives as a novel class of Farnesoid X Receptor (FXR) agonists. Bioorganic & Medicinal Chemistry. 20 (11), 3429-3445 (2012).
- Ochiai, H., et al. Discovery of new orally available active phosphodiesterase inhibitors. Chemical and Pharmaceutical Bulletin. 52 (9), 1098-1104 (2004).
- Ganesan, A., Heathcock, C. H. Synthesis of unsymmetrical pyrazines by reaction of an oxadiazinone with enamines. Journal of Organic Chemistry. 58 (22), 6155-6157 (1993).
- Sumesh, R. V., et al. Multicomponent dipolar cycloaddition strategy: combinatorial synthesis of novel spiro-tethered pyrazolo[3,4-b]quinoline hybrid heterocycles. ACS Combinatorial Science. 18 (5), 262-270 (2016).
- Bagley, M. C., Davis, T., Dix, M. C., Widdowson, C. S., Kipling, D. Microwave-assisted synthesis of N-pyrazole ureas and the p38α inhibitor BIRB 796 for study into accelerated cell ageing. Organic & Biomolecular Chemistry. 4 (22), 4158-4164 (2006).
- Su, W., Lin, T., Cheng, K., Sung, K., Lin, S., Wong, F. An efficient on-pot synthesis of N-(1,3-diphenyl-1H-pyrazol-5-yl)amides. Journal of Heterocyclic Chemistry. 47 (4), 831-837 (2010).
- Eagon, S., Anderson, M. O. Microwave-assisted synthesis of tetrahydro-β-carbolines and β-carbolines. European Journal of Organic Chemistry. (8), 1653-1665 (2014).
- Everson, N., et al. Microwave synthesis of 1-aryl-1H-pyrazole-5-amines. Tetrahedron Letters. 60 (1), 72-74 (2019).

