A Simple Neuronal Mechanical Injury Methodology to Study Drosophila Motor Neuron Degeneration
Summary
Here we describe a simple and widely accessible method to injure segmental nerves in Drosophila larvae to visualize and quantify neurodegeneration of motor neurons at the neuromuscular junction (NMJ) of third instar larvae.
Abstract
The degeneration of neurons occurs during normal development and in response to injury, stress, and disease. The cellular hallmarks of neuronal degeneration are remarkably similar in humans and invertebrates as are the molecular mechanisms that drive these processes. The fruit fly, Drosophila melanogaster, provides a powerful yet simple genetic model organism to study the cellular complexities of neurodegenerative diseases. In fact, approximately 70% of disease-associated human genes have a Drosophila homolog and a plethora of tools and assays have been described using flies to study human neurodegenerative diseases. More specifically the neuromuscular junction (NMJ) in Drosophila has proven to be an effective system to study neuromuscular diseases because of the ability to analyze the structural connections between the neuron and the muscle. Here, we report on an in vivo motor neuron injury assay in Drosophila, which reproducibly induces neurodegeneration at the NMJ by 24 h. Using this methodology, we have described a temporal sequence of cellular events resulting in motor neuron degeneration. The injury method has diverse applications and has also been utilized to identify specific genes required for neurodegeneration and to dissect transcriptional responses to neuronal injury.
Introduction
Neuronal degeneration occurs during normal development and can be caused by the natural aging process, injury, stress, or disease states. Drosophila melanogaster, the common fruit fly, provides a simple and powerful model organism to study neurodegeneration due to the remarkable similarities in the molecular mechanisms that drive the degeneration of neurons. These similarities are highlighted by the fact that approximately 70% of disease-associated human genes have a Drosophila homolog.1 Additionally, numerous assays and technological tools to study human neurodegenerative diseases have been developed and utilized in Drosophila.2,3 Within Drosophila, the neuromuscular junction (NMJ) allows for analysis of both cellular and electrophysiological properties and has proven to be an important system to study neuromuscular disease due to the visible neuron-muscle connections.2 In this study, we describe an in vivo neuron injury assay in Drosophila larvae which allows for the reproducible injury of segmental nerves. This motoneuron injury results in a temporal sequence of cellular events resulting in neurodegeneration at the NMJ 24 h post injury. The ability to reproducibly injury motoneurons resulting in neurodegeneration has diverse applications such as identifying specific genes required for the degenerative process, the dissection of transcriptional responses to neuronal injury, and the analysis of protective signaling cascades.4,5,6 This method has also been used in combination with microfluidics to study neuronal degeneration and regeneration in live animals.7
We utilize an established quantitative assay to examine motor neuron degeneration at the Drosophila NMJ after mechanical injury. This assay is based on the fact that loss of presynaptic membrane and proteins precedes the disassembly of the subsynaptic reticulum (SSR) characterized by the postsynaptic muscle membrane folds.8,9,10,11,12,13 This assay allows for the quantification of "synaptic footprints" where the pre-synaptic neuron has lost connection to the adjacent postsynaptic muscle. The degenerative process has been shown to be progressive throughout larval development12 and cannot be accounted for by altered synapse development or sprouting.8,9,10,11,12 The advantage of using mechanical injury over preexisting mutations is that it allows for dissection of the temporal sequence of cellular events leading up to neurodegeneration at the NMJ.13
Protocol
1. Preparation of Reagents and Equipment
- Prepare 1x Dissection Buffer (70 mM NaCl, 5 mM KCl, 0.02 mM CaCl2, 20 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 5 mM trehalose, 5 mM HEPES; pH 7.2).
- Prepare 1x Phosphate-Buffered Saline (PBS).
- Prepare 1x PBT using 1x PBS with 0.01% Triton X-100.
- Prepare fruit juice agar plates.14 Briefly, mix 30 g agar in 700 mL of H2O and autoclave. Dissolve 0.5 g of methyl paraben in 10 mL of ethanol and add solution to 300 mL of fruit juice concentrate (grape or apple). Mix concentrate into autoclaved agar solution and pour into the lids of 10 mm x 35 mm Petri dishes.
NOTE: Grape agar power premix can also be purchased from various companies (see Tabls of Materials). - Obtain size 5 forceps that allow one to mechanically injure the larvae.
- Use standard Drosophila CO2 based anesthesia apparatus.
2. Selecting Drosophila Larvae
- Take a vial or bottle of Drosophila containing wandering third instar larvae that have been cultured at 25 °C.
- Select ten individual 2nd or 3rd instar larvae based on appearance. Third instar larvae can be identified by the appearance of anterior branched spiracles, while second instar larvae are smaller and have more club-like anterior spiracles.
NOTE: Larval stages can also be identified by timing. At 25 °C, second instar larvae appear approximately 48 h after hatching, and third instar larvae molt approximately 24 h later. If interested in examining neurodegeneration at the NMJ, second instar larvae need to be injured.
3. Preparing Drosophila Larvae for Mechanical Injury
- Carefully pick individual larvae up by forceps or a paintbrush, being cautious not to compress it in anyway.
- Directly place ten larvae into glass dish containing cold 1X PBS or dissection buffer to remove any food debris and to decelerate larval motility.
4. Mechanical Injury and Treatment of Drosophila Larvae
- Place each individual larva onto a CO2 anesthetizing apparatus.
- Under a dissecting microscope, carefully roll each larva onto their dorsal side in order to visualize the segmental nerves through the cuticle.
- Position size 5 forceps approximately 1/2 to 2/3 way down the length of the larva starting at the mouth hooks. Once positioned, pinch approximately 1/3 of the ventral cuticle containing the segmental nerves with size 5 forceps.
- To ensure that larval segmental nerves are injured, apply sufficient force to crush the segmental nerves but leave the cuticle intact. To determine the correct amount of force, crush 10 larvae and ensure the death rate after 5 h is under 50%.
- After injury, carefully transfer larvae to a fruit juice agar plate containing approximately 0.5 g of yeast paste. Place each larva so its anterior end is on the yeast paste, for continued feeding despite disrupted motility.
NOTE: To ensure that larval segmental nerves have been injured, disrupted larval motility can be observed after transfer to the agar plate. Injured larva are effectively paralyzed posterior to the site of injury, but can still move their mouth hooks and feed. A high death rate of animals after injury is common so it is best to injure 20-50% more animals than is necessary for an experiment. - Place agar plates containing yeast paste and 10 injured larvae into the bottom of an empty 100 x 15 mm petri dish. Cover this petri plate, now containing the injured larvae on an agar plate with a moist paper towel, ensuring that the paper towel does not touch the larvae or the agar plates. Place this Petri dish into a larger air-tight container, at RT.
- Retrieve larvae for dissection after specified periods (6, 12, or 24 h) post-injury.
NOTE: To examine immediate effects of injury on the axonal cytoskeleton, larvae can be dissected directly after injury. To examine axonal transport defects, larvae can be dissected approximately 6 h after injury. About 12 h after injury a build-up of ubiquitinated proteins can be observed, and 24 h after injury, degeneration of motor neurons at the NMJ can be observed.
5. Analysis of Neurodegeneration at the NMJ
- Dissect Drosophila larval NMJ as described previously.15,16 Briefly, pin larvae onto a Sylgard plate, cut along the dorsal surface, and fillet with pins in a drop of dissection buffer to expose the body wall musculature. Fix larvae in either 4% paraformaldehyde (PFA) or Bouin's Solution depending on the antibodies used to visualize neurons and muscle.
NOTE: PFA fixation should be used if fluorescent protein tags, such as Green Fluorescent Protein (GFP) is to be visualized as Bouin's fixative can be too harsh. - Perform immunofluorescence, which has been described previously11,13,16, to simultaneously mark presynaptic motor neurons and adjacent postsynaptic (SSR) muscle folds. Visualize presynaptic membranes and axons with fluorescently conjugated horseradish peroxidase (HRP) (1:300)17 and active zones can be stained using an anti-Bruchpilot (Brp) antibody (nc82; 1:100; Developmental Studies Hybridoma Bank). Simultaneously label post-synaptic muscles with an anti-Dics Large (Dlg) antibody (1:10,000).18
- Perform an established assay for quantifying neurodegeneration at the NMJ as described previously.8,9,10,11,12,13,14 Briefly, simultaneously visualize individual NMJs for both pre- and post-synaptic compartments. Any boutons labeled with postsynaptic markers but not presynaptic markers indicate sites of neurodegeneration (Figure 1). The average number of boutons per NMJ as well as the average number of NMJs per animal can be quantified.
Representative Results
Using the procedure presented here, we have demonstrated that mechanical neuronal injury allows for temporal dissection of neurodegenerative events.14,18 The sequence of events has been previously characterized and begins with an immediate disruption of the cytoskeleton, followed by axonal trafficking defects, an accumulation of ubiquitinated proteins, and subsequent neurodegeneration 24 h post-injury. Prior to injury, WT NMJs show the presynaptic active zone marker Brp in apposition to the postsynaptic marker Dlg throughout the entire NMJ (Figure 1A). Mechanical injury of segmental nerves can induce moderate to severe neurodegeneration in which boutons stained with Dlg now lack the presynaptic active zone protein Brp (Figure 1B). Any bouton that is stained with Dlg but lacks Brp staining is considered a "synaptic footprint" and can be counted and quantified as a neurodegenerative event. Both the frequency and severity of the neurodegenerative phenotype can be quantified as described previously.11
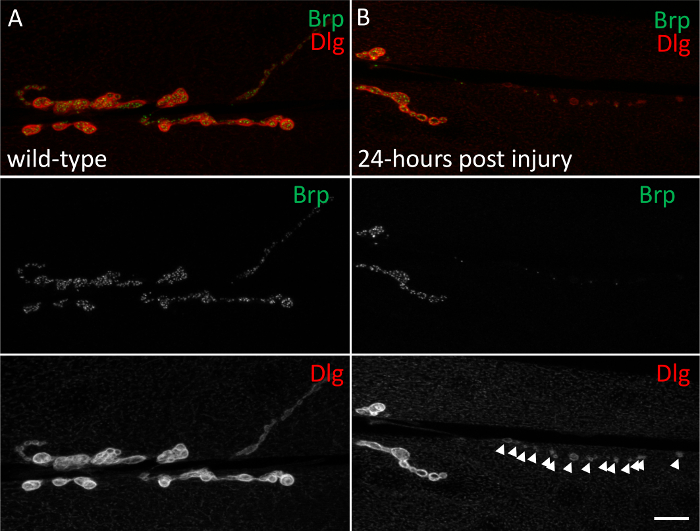
Figure 1: Mechanical neuronal injury induces neurodegeneration at the NMJ of muscle 6/7. (A) Uninjured NMJs show the pre-synaptic active zone marker, Brp (green) in apposition with the postsynaptic marker, Dlg (red) throughout the entire NMJ. (B) Neuronal mechanical injury induces neurodegeneration indicated by Dlg stained boutons (red) with the loss of Brp. Arrowheads indicate individual sites of neurodegeneration. Scale bar = 10 µm. Please click here to view a larger version of this figure.
Discussion
The neuronal mechanical injury described earlier and demonstrated here can be used to induce injury/stress in the segmental nerves of Drosophila larvae.4,5,6,14 This experimental technique has been employed previously to dissect the temporal sequence of events leading to neurodegeneration, as well as to examine transcriptional changes in the motor neuron cell bodies post injury.5,14 Additionally, this technique has been described in conjunction with microfluidics chips in order to observe and study axonal degeneration and regeneration in Drosophila larvae.7 Limitations of this technique include death of the larvae due to puncturing of the cuticle during introduction of the injury. However, a large number of larvae can be crushed in a short period of time to overcome a high larval death rate. A modification that we have included is the injury of 2nd instar or early 3rd instar larvae, which is critical to visualize motor neuron degeneration at the NMJ, which occurs 24 h post injury.
Here, we demonstrate that neuronal mechanical injury can be utilized to study motor neuron degeneration at the NMJ. We use an established method to quantify neurodegeneration that takes advantage of the fact that neurons and their associated proteins degenerate more rapidly than the adjacent muscle.8,9,10,11,12,13,14 To observe and quantify neurodegeneration, it is critical that the NMJ be simultaneously stained for both pre- and postsynaptic markers. Here, we demonstrate the use of the active zone marker Brp and the postsynaptic marker Dlg, however, there are a plethora of other pre- and postsynaptic markers available that may be utilized to study neurodegeneration at the NMJ. Additionally, fluorescently-tagged molecules of one's choosing may be employed to study their effects during the neurodegenerative process. There are more complicated methods to study degeneration after axonal injury, such as microfluidics7, however our method has several advantages: 1) it is very inexpensive, 2) most Drosophila labs already have the necessary equipment, and 3) the tissue is fixed so conventional fluorescence microscopy can be used to quantify neurodegenerative events post injury.
We envision that this protocol may be adapted to examine a wide-range of cellular and molecular mechanisms related to neurodegeneration after injury. In particular, these methods could be used to examine the behavior of any fluorescently-tagged protein before and after neuronal injury and motor neuron degeneration and regeneration.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank all members of the Keller and Magie Labs at Quinnipiac University for helpful suggestions. In particular, we would like to thank Barron L. Lincoln II for development of this injury assay within the Keller Lab. We would also like to thank Quinnipiac University College of Arts and Science Grant-In-Aid awarded to L.C. Keller.
Materials
| Micro-dissecting scissors | Fine Science Tools | 15000-08 | |
| Dumont #3 Forceps | Fine Science Tools | 11231-30 | Some people prefer size 3, while others prefer size 5 |
| Dumont #5 Forceps | Fine Science Tools | 11251-30 | Some people prefer size 3, while others prefer size 5 |
| CO2 Air Tank | Tech Air | UN 1013 | Various tank sizes can be purchased/ |
| CO2 Anesthetizing Apparatus | Genesee Scientific | 59-114 | |
| Stainless-steel pins, size 0.1 | Fine Science Tools | 26002-10 | |
| SylGard 184 Silicone Elastomer, Base and Curing Agent | Dow Corning | 3097358-1004 | To pour dissecting plates |
| Bouin's Solution | Sigma | HT 10132-1L | Antibodies should be tested for their efficiency in Bouin's and PFA |
| 4% Paraformaldehyde in PBS | Affymetrix | FLY-8030-20 | Antibodies should be tested for their efficiency in Bouin's and PFA |
| Dissecting Stereo MIcroscope | AmScope | SM-1BZ | |
| Light Source | AmScope | HL150-AY-220V | |
| anti- nc82 antibody | Developmental Studies Hybridoma Bank | nc82-s | |
| anti-discs large antibody | Developmental Studies Hybridoma Bank | AF3 | |
| Alexa Fluor anti-horseradish peroxidase | Jackson Immunoresearch | 123-545-021; 123-585-021; 123-605-021 | One can you Alexa Fluor® 488, 594 or 647 |
| Flystuff Grape Juice Agar Premix | Genesee Scientific | 47-102 | |
| Microscope slides | Genesee Scientific | 29-101 | |
| Glass Coverslips | Fisher Scientific | 12-545-87 | |
| Thermo Scientific Nalgene Utility Box | Fisher Scientific | 03-484C | Used to create humid chamber for larval recovery |
Referências
- Bier, E. Drosophilia, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 6 (1), 9-23 (2005).
- Ugur, B., Chen, K., Bellen, H. J. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9 (3), 235-244 (2016).
- McGurk, L., Berson, A., Bonini, N. M. Drosophila as an in vivo model for human neurodegenerative disease. Genética. 201 (2), 377-402 (2015).
- Shin, J. E., Cho, Y., Beirowski, B., Milbrandt, J., Cavalli, V., DiAntonio, A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 74 (6), 1015-1022 (2012).
- Xiong, X., Wang, X., Ewanek, R., Bhat, P., Diantonio, A., Collins, C. A. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 191 (1), 211-223 (2010).
- Xiong, X., Collins, C. A. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci. 32 (2), 610-615 (2012).
- Mishra, B., Ghannad-Rezaie, M., Li, J., Wang, X., Hao, Y., Ye, B., Chronis, N., Collins, C. A. Using microfluidics chips for live imaging and study of injury responses in Drosophila larvae. J Vis Exp. (84), e50998 (2014).
- Eaton, B. A., Fetter, R. D., Davis, G. W. Dynactic is necessary for synapse stabilization. Neuron. 34 (5), 729-741 (2002).
- Eaton, B. A., Davis, G. W. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 47 (5), 695-708 (2005).
- Pielage, J., Fetter, R. D., Davis, G. W. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 15 (10), 918-928 (2005).
- Pielage, J., Cheng, L., Fetter, R. D., Carlton, P. M., Sedat, J. W., Davis, G. W. A presynaptic giant Ankyrin stabilizes the NMJ through regulation or presynaptic microtubules and transsynaptic cell adhesion. Neuron. 58 (2), 195-209 (2008).
- Massaro, C. M., Pielage, J., Davis, G. W. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankryin/microtubule cytoskeleton. J Cell Biol. 187 (1), 101-117 (2009).
- Lincoln, B. L., Alabsi, S. H., Frendo, N., Freund, R., Keller, L. C. Drosophila neuronal injury follows a temporal sequence of cellular events leading to degeneration at the neuromuscular junction. J Exp Neurosci. 9, 1-9 (2015).
- . Drosophila apple juice-agar plates. Cold Spring Harb Protoc. , (2011).
- Brent, J. R., Werner, K. M., McCabe, B. D. Drosophila larval NMJ immunohistochemistry. J Vis Exp. (25), (2009).
- Smith, R., Taylor, J. P. Dissection and imaging of active zones in the Drosophila neuromuscular junction. J Vis Exp. (50), (2011).
- Jan, L., Jan, Y. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. 79 (8), 2700-2704 (1982).
- Lahey, T., Gorczyca, M., Jia, X., Budnik, V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 13 (4), 823-835 (1994).

