Generation of Human Adipose Stem Cells through Dedifferentiation of Mature Adipocytes in Ceiling Cultures
Summary
Mature adipocytes may represent an abundant source of stem cells through dedifferentiation, which leads to a homogenous population of fibroblast-like cells. Collagenase digestion is used to isolate mature adipocytes from human fat. The goal of our protocol is to obtain multipotent, dedifferentiated fat cells from human mature adipocytes.
Abstract
Mature adipocytes have been shown to reverse their phenotype into fibroblast-like cells in vitro through a technique called ceiling culture. Mature adipocytes can also be isolated from fresh adipose tissue for depot-specific characterization of their function and metabolic properties. Here, we describe a well-established protocol to isolate mature adipocytes from adipose tissues using collagenase digestion, and subsequent steps to perform ceiling cultures. Briefly, adipose tissues are incubated in a Krebs-Ringer-Henseleit buffer containing collagenase to disrupt tissue matrix. Floating mature adipocytes are collected on the top surface of the buffer. Mature cells are plated in a T25-flask completely filled with media and incubated upside down for a week. An alternative 6-well plate culture approach allows the characterization of adipocytes undergoing dedifferentiation. Adipocyte morphology drastically changes over time of culture. Immunofluorescence can be easily performed on slides cultivated in 6-well plates as demonstrated by FABP4 immunofluorescence staining. FABP4 protein is present in mature adipocytes but down-regulated through dedifferentiation of fat cells. Mature adipocyte dedifferentiation may represent a new avenue for cell therapy and tissue engineering.
Introduction
In vitro dedifferentiation of mature adipocytes is achieved through a technique called ceiling culture1. Because of their natural tendency to float in aqueous solutions, isolated mature adipocytes adhere to the surface of an inverted flask fully filled with culture medium. Over a few days, cells modify their spherical morphology and become fibroblast-like cells. The resulting cells, called dedifferentiated fat (DFAT) cells, are multipotent2. Research articles on adipocyte dedifferentiation, especially on human cells, are limited. However, they have already provided interesting information regarding multipotency, cell phenotype and replicative capacity of DFAT cells2. Mature adipocytes originating from various fat compartments have been successfully dedifferentiated including those originating from human visceral and subcutaneous adipose tissues2-4. In addition to these depots, Kishimoto and collaborators sampled adipose tissue from the buccal fat pads and dedifferentiated adipocytes into DFAT cells5. Matsumoto and collaborators successfully generated subcutaneous DFAT cells from patients covering a wide range of ages, and the majority of cells had a high proliferative rate and less than 6% of senescence even after 10 passages in culture2.
DFAT cells have been successfully re-differentiated into several lineages, including adipogenic, osteogenic, chondrogenic and neurogenic lineages2,3,6. These cells express several embryonic stem cell markers such as Nanog and the four identified pluripotent factors Oct4, c-myc, Klf4 and Sox23. They also express markers specific to each of the three germ layers7. In addition, DFAT cells are similar to Bone Marrow-derived Mesenchymal Stem Cells (BM-derived MSC) based on their epigenetic signature3. Exploiting the stem cell capacity of DFAT cells, many groups have investigated their potential to treat or improve various diseases8,9. Improvements of pathologic conditions, such as infracted cardiac tissue, spinal cord injury and urethral sphincter dysfunction, have been observed with DFAT cell injections in rat models of disease10-12.
In addition to the stem cell properties of DFAT cells, they may represent a new cellular model for adipocyte physiology studies. The 3T3-L1 cell line is often used for this purpose as these cells differentiate into adherent, lipid-storing adipocytes under adipogenic stimulation13. However, these cells originate from mouse embryo tissue13. Also, depot-specificity cannot be investigated with this model and it may not fully reflect human adipocyte physiology14. Other laboratories work with isolated adipose cells from murine fat depots, but fat distribution is not dimorphic in mice and anatomical configuration of the rodent’s abdominal cavity prevents from extrapolating directly to humans15. In order to study adipocytes in the context of the physiopathology of human obesity, consideration of body fat distribution and fat depot-specific differences has become essential16. Some limitations of primary preadipocyte cultures, including cell quantities obtained from adipose tissue biopsy samples and their senescence after a few passages in culture, created the need for alternate models. Perrini and collaborators investigated depot-specificity in gene expression of DFAT cells originating from visceral and subcutaneous fat and compared them to adipose-derived stem cells (ASC) from the same fat depot. They demonstrated that differences in gene expression and function where mainly found between depots than between cell types, suggesting that DFAT cells are physiologically close to ASC from the same depot. DFAT cells may represent an interesting alternative to available models for studies on fat distribution in the pathophysiology of human obesity. Moreover, ceiling culture is a promising method to obtain adult stem cells for tissue engineering purposes.
Here, we describe collagenase digestion, a widely-used technique to isolate mature adipocytes from the subcutaneous and/or visceral fat samples17, and the subsequent steps to perform ceiling culture and dedifferentiate these cells into multipotent, fibroblast-like cells.
Protocol
Ethics statement: The project has been approved by IUCPQ’s Research Ethics Committee prior to patient recruitment. For the purpose of this article/video, we obtained tissues from 2 patients: 1) a 62 year-old male patient with a BMI of 50.7 kg/m2 and 2) a 35 year-old female patient with a BMI of 57 kg/m2. Experiments can be done with both fat compartments, but have been limited to one fat compartment for the purpose of this video. Technical aspects of the video were performed with patient 1 and FABP4 immunofluorescence was performed with dedifferentiated cells from patient 2.
1. Sample Processing
- Ask surgeons to collect adipose tissue from the omental and subcutaneous fat compartments at the time of laparoscopic bariatric surgery.
- Quickly bring adipose samples to the laboratory at RT and process immediately.
- Perform digestion in the laboratory, in a non-sterile atmosphere. The cells will eventually be transferred to the culture room and cultivated under sterile conditions. To avoid contamination, prepare KRH buffer with distilled and filtrated water and follow by a filtration (0.22µM filter) prior to digestion. Thoroughly clean tubes with ethanol prior transfer in the cell culture hood for flask and plate preparation.
- Place adipose tissue on a pre-weighted dish and record weight. Fix a small piece of each tissue sample (less than 1 cm2) in 10% formalin buffer at RT for at least 24 hr before paraffin embedding. Use this embedded sample for immunohistochemistry experiments (technique not shown).
- Place another piece in a 50-ml tube and flash-freeze in liquid nitrogen before storing at -80 °C for further studies on whole adipose tissues (e.g., gene expression – technique not shown).
2. Collagenase Digestion
- Place the remaining adipose tissue piece in a 50 ml tube for digestion.
- Add 4 ml of KRH-WB supplemented with collagenase (350 U/ml) per gram of sample in the digestion tube.
- Mince adipose tissue with scissors.
- Place minced adipose tissue suspension in a shaker, 37 °C, 90 rpm maximum, for a 45-minute incubation (maximum 1 hr).
3. Purification of Adipocytes and Preadipocytes
- Pour the translucent solution with few chunks of fat through a 400 µM nylon mesh into a plastic beaker.
- With tweezers, rub the cell preparation on the nylon mesh and wash with 5 ml of KRH-WB.
- Delicately transfer the filtrated cell suspension into a 50 ml tube with the plastic tubing in it and a 60cc syringe attached at the tubing extremity.
- Let the suspension with mature adipocytes stand for approximately 10 min, allowing the cells to reach the top of the buffer by floatation.
- Slowly aspirate the buffer at the bottom of the tube using 60cc syringe suction.
- Add 20 ml of KRH-WB to wash. Repeat from step 3.4 for 2 additional washes.
- Collect the buffer to bring the adipocyte suspension to a final volume of 5 or 10 ml, depending on cell quantity. Pursue with steps in section 5.
- Recover the stromal-vascular fraction from the buffer collected with the 60cc syringe by centrifugation (3,000 rpm, RT, 5 min) for further primary cell culture if desired (technique not shown).
4. Mature Adipocyte Cell Count
- Load 10 µl of gently shaken adipocyte suspension in a counting chamber (haemocytometer). Perform cell count in quadruplicate.
- Calculate number of isolated mature cells.
5. Mature Adipocyte Dedifferentiation into T-25 Flask
- Fill a 25 cm2 tissue culture flask to ¾ of the volume with DMEM/F12-20% calf serum.
- According to cell count, pour 500,000 mature cells into the flask.
- Fill the flask completely using a 50ml tube with medium and remove as many bubbles as possible.
- Screw the unvented cap on the flask.
- Clean the flask with ethanol prior to incubation to avoid contamination.
- Incubate the flask upside down for a week without touching it to avoid movement in the culture that may disrupt cellular adherence.
- Prior to reversing the flask at 7 days of inverted culture, gently manipulate the flask and remove all medium in the flask by aspiration, avoiding abrupt movements.
- Add 12 ml of DMEM-F12-20% calf serum and cultivate cells with standard techniques. A filtered, vented cap may be added to the flask.
6. Mature Adipocyte Dedifferentiation into a 6-well Plate
- Place a coverslip on the bottom of each well of a 6-well plate
- Add a ½” plastic bushing on top of each coverslip.
- Fill wells with 8 ml of 20% calf serum-DMEM medium.
- Put a coverslip on each plastic bushing.
- Insert pipet tip between the slide and the tube to inject cells under the slide (50,000 cells per well).
- Incubate plates in a standard cell culture incubator at 37 °C with 5% CO2 for a week.
- Reverse coverslip with attached cells into each well containing 2 ml of media supplemented with 20% calf serum and pursue culture.
- Use coverslip with cells undergoing dedifferentiation for several purposes including immunofluorescence (technique not shown).
Representative Results
Major morphological changes occur to mature adipocytes during dedifferentiation (Figure 1). As shown in Figure 2, cells undergoing dedifferentiation were stained with an anti-FABP4 antibody for fluorescence analysis. Cells with a round morphology expressed the FABP4 protein whereas the majority of the fibroblast-like cells did not. After dedifferentiation, DFAT cells can be cultivated with standard procedures for several passages. We have been able to reach more than 15 passages for human omental and subcutaneous DFAT cell lines (data not shown).
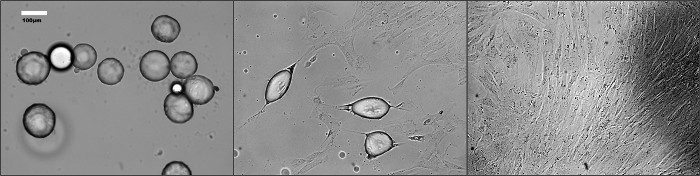
Figure 1. Morphology of dedifferentiating mature adipocytes over time (A) at 4 days (B) at 7 days and (C) at 12 days of culture. Pictures were taken at different time-points during the incubation using a phase-contrast microscope. Please click here to view a larger version of this figure.
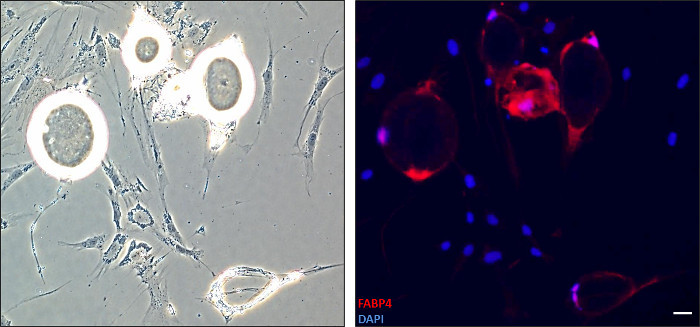
Figure 2. Detection of FABP4 protein in adipocytes undergoing dedifferentiation. Cells were fixed after 13 days of dedifferentiation and stained with anti-FABP4 antibody for immunofluorescence. Nuclei were visualized with DAPI staining. Left: Brightfield image of corresponding immunofluorescence. Right: The merged image is shown (FABP4-red, Nuclei-blue-10X). Adipocytes with round morphology express FABP4, a mature adipocyte marker, whereas elongated cells no longer express it. Scale bar: 1 unit = 0.25mm Please click here to view a larger version of this figure.
Discussion
Dedifferentiation of mature adipocytes with the ceiling culture technique is a new approach to obtain adipose stem cells from a small sample of native adipose tissue. Based on our experience and that of others2, one gram of tissue is sufficient to plate a 25-cm2 flask and to obtain a population of DFAT cells for which homogeneity has been demonstrated by Poloni and collaborators3. Adipocyte dedifferentiation seems possible with cells from any donor, independently of their age, sex and other characteristics. Among the resulting population of DFAT obtained, there remains a few round or partly elongated cells that did not fully dedifferentiate. These cells are usually discarded through the culture passage as they float in the trypsin-media mix.
Multipotency of these cells is established and supports their use for cell therapy2,3. Their high proliferative capacity has also been reported, which is a valuable aspect of cell culture for stem cell applications2. Studies with human DFAT cells indicated that they may be more efficient than ASC from the same donor, based on their replicative and differentiation capacity18. A recent case study supports that DFAT cells were more efficient to differentiate into adipocytes and osteoblasts, and had higher telomerase levels than ASC from the same individual, a donor with obesity and diabetes18. Thus, the use of ceiling culture may provide more efficient adipose stem cells than the already used ASC. However, additional experiments are needed to clearly assess this point.
Our 6-well plate ceiling culture technique allows for the study of the dedifferentiation process itself. A minimal number of cells can be plated and allows for the study of specific time-points. For example, we collected the microscope slide from a 6-well plate to perform immunofluorescence from adipocytes undergoing dedifferentiation (Figure 2). Performing miscroscopy, with or without fluorescence, is highly relevant to assess various aspects of dedifferentiation.
In addition to stem cell applications, DFAT cells may represent an interesting model for physiological studies. Only a few studies examined gene expression and functions of both cell types. In brief, ASC and DFAT from the same fat compartment showed similarities in gene expression and secretion4. More comparisons between ASC and DFAT from the same donor are necessary.
In conclusion, we show in this technical report how to obtain DFAT cells from human adipose tissue using the well-established technique of adipose tissue collagenase digestion and the ceiling culture technique. Our original 6-well plate format may help increase knowledge on the dedifferentiation process whereas the more commonly used flask method allows for the generation of larger populations of DFAT cells. The major limitation of this protocol is the access to human adipose tissue which relies on collaboration with a surgery team and ethics management to obtain patient written and informed consent. Mature cells are highly sensitive which requires precautions in the manipulation. We optimized the protocol, especially the number of adipocytes to be plated in the T-25flask and the 6-well plate format, to obtain optimal results and no major additional modifications or troubleshooting may be anticipated.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by Natural Sciences and Engineering Research Council of Canada Discovery Grant (371697-2011, AT). The authors want to acknowledge the help of bariatric surgeons Drs S. Biron, F-S. Hould, S. Lebel, O. Lescelleur, P. Marceau as well as Christine Racine and Caroline Gagnon from the IUCPQ Tissue Bank. We thank Mr Jacques Cadorette from the IUCPQ’s audiovisual services for video shooting and editing.
Materials
| Bovine serum albumine | Sigma | A7906 | |
| Adenosine | Sigma | A4036 | |
| Ascorbic acid | Sigma | A0278 | |
| NaCl | Any brand can be used | ||
| KCl | Any brand can be used | ||
| CaCl2 | Any brand can be used | ||
| MgCl2 | Any brand can be used | ||
| KH2PO4 | Any brand can be used | ||
| HEPES | Any brand can be used | ||
| Glucose | Any brand can be used | ||
| Type I collagenase | Worthington Biochemical Corp | LS-004196 | |
| DMEM/F-12, HEPES, no phenol red | Gibco-Life Technologies | 11039-021 | Add to medium : 20% calf serum, gentamicin (50µg/ml) and fungizone (2.5µg/ml) |
| Calf Serum, iron supplemented, from formula-fed calves | Sigma | C8056-500ml | |
| 1/2 In plastic bushing | Iberville | 2704-CP | SKU:1000120918 (Home Depot) |
| Liquid nitrogen | Linde | ||
| Formalin soluton, neutral buffered, 10% | SIGMA | HT501128 | |
| Sterile tweezers | |||
| Sterile scissors | |||
| 60cc syringes | BD Syringe | ||
| Plastic tubing | |||
| Krebs-Ringer-Henseleit stock buffer (KRH) | Prepare stock buffer as following: 25mM HEPES pH7.6, 125mM NaCl, 3.73mM KCl, 5mM CaCl2.2H2O, 2.5mM MgCl2.6H2O, 1mM K2HPO4. Adjust pH to 7.4. | ||
| Krebs-Ringer-Henseleit-Working Buffer (KRH-WB) | Add the following components freshly to KRH buffer: 4% bovine serum albumin, 5mM glucose, 0.1µM adenosine, 560 µM ascorbic acid | ||
| KRH-WB supplemented with Type I collagenase | Add 350U/ml of Type I collagenase | ||
| T25 unvented cap tissue culture flask | Sarsted or other brand | ||
| 6-well tissue culture plate | BD Falcon or other brand | ||
| Microscope cover glass 22×22 | Fisherbrand | 12-542-B | |
| Sterile beakers |
Referências
- Zhang, H. H., Kumar, S., Barnett, A. H., Eggo, M. C. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 164 (2), 119-128 (2000).
- Matsumoto, T., et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 215 (1), 210-222 (2008).
- Poloni, A., et al. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 30 (5), 965-974 (2012).
- Perrini, S., et al. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 8 (3), e57892 (2013).
- Kishimoto, N., et al. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin Oral Investig. , (2013).
- Kou, L., et al. The phenotype and tissue-specific nature of multipotent cells derived from human mature adipocytes. Biochem Biophys Res Commun. 444 (4), 543-548 (2014).
- Jumabay, M., et al. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl Med. 3 (2), 161-171 (2014).
- Sugawara, A., Sato, S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Hum Cell. 27 (1), 12-21 (2014).
- Kikuta, S., et al. Osteogenic effects of dedifferentiated fat cell transplantation in rabbit models of bone defect and ovariectomy-induced osteoporosis. Tissue Eng Part A. 19 (15-16), 1792-1802 (2013).
- Obinata, D., et al. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 18 (12), 827-834 (2011).
- Jumabay, M., et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 47 (5), 565-575 (2009).
- Ohta, Y., et al. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 17 (8), 877-886 (2008).
- Moreno-Navarrete, J. M. F. -. r., Symonds, M. E. Ch. 2. Adipose Tissue Biology. , 17-38 (2012).
- Poulos, S. P., Dodson, M. V., Hausman, G. J. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood. 235 (10), 1185-1193 (2010).
- Casteilla, L., Penicaud, L., Cousin, B., Calise, D. Choosing an adipose tissue depot for sampling: factors in selection and depot specificity). Methods Mol Biol. 456, 23-38 (2008).
- Tchernof, A., Despres, J. P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 93 (1), 359-404 (2013).
- Rodbell, M. Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J Biol Chem. 239, 375-380 (1964).
- Watson, J. E., et al. Comparison of Markers and Functional Attributes of Human Adipose-Derived Stem Cells and Dedifferentiated Adipocyte Cells from Subcutaneous Fat of an Obese Diabetic Donor. Adv Wound Care. 3 (3), 219-228 (2014).

