Purification of the Cystic Fibrosis Transmembrane Conductance Regulator Protein Expressed in Saccharomyces cerevisiae
Summary
Heterologous expression and purification of the cystic fibrosis transmembrane conductance regulator (CFTR) are significant challenges and limiting factors in the development of drug therapies for cystic fibrosis. This protocol describes two methods for the isolation of milligram quantities of CFTR suitable for functional and structural studies.
Abstract
Defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein cause cystic fibrosis (CF), an autosomal recessive disease that currently limits the average life expectancy of sufferers to <40 years of age. The development of novel drug molecules to restore the activity of CFTR is an important goal in the treatment CF, and the isolation of functionally active CFTR is a useful step towards achieving this goal.
We describe two methods for the purification of CFTR from a eukaryotic heterologous expression system, S. cerevisiae. Like prokaryotic systems, S. cerevisiae can be rapidly grown in the lab at low cost, but can also traffic and posttranslationally modify large membrane proteins. The selection of detergents for solubilization and purification is a critical step in the purification of any membrane protein. Having screened for the solubility of CFTR in several detergents, we have chosen two contrasting detergents for use in the purification that allow the final CFTR preparation to be tailored to the subsequently planned experiments.
In this method, we provide comparison of the purification of CFTR in dodecyl-β-D-maltoside (DDM) and 1-tetradecanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (LPG-14). Protein purified in DDM by this method shows ATPase activity in functional assays. Protein purified in LPG-14 shows high purity and yield, can be employed to study post-translational modifications, and can be used for structural methods such as small-angle X-ray scattering and electron microscopy. However it displays significantly lower ATPase activity.
Introduction
Cystic fibrosis (CF) is the most common genetic disorder in Europe and North America with an incidence of about 1 in 2,500 live births. CF occurs when mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein cause loss of its function at the plasma membrane of epithelial cells1. The most serious consequence of this defect is irreversible lung damage, which shortens the life expectancy of sufferers to <40 years of age2,3.
CFTR is an ATP-binding cassette (ABC) transporter that has evolved to become an ion channel1,4. Despite its quite altered function in the plasma membrane of cells, it still retains significant sequence homology with other ABC transporters. Intriguingly, the specialized parts of CFTR (i.e. its regulatory region and its N- and C-termini) share no significant sequence similarity with other metazoan ABC transporters, hence there are no clues as to the origins of these sequences in CFTR. On the basis of its primary structure, CFTR is classified as a C-family member of the ABC transporter family, but there is no strong evidence for a residual functional linkage to this sub-family. There have been some reports of glutathione transport activity for CFTR5-7, which would be consistent with the roles of other C-family members8,9, although other reports suggest that reduced glutathione may inhibit the CFTR ATPase activity, rather than showing the substrate-induced stimulation that characterize the ABC transporters10. Measurement of ion conductance is sufficiently sensitive to allow the channel activity of single CFTR molecules to be studied1 and CFTR channel properties have been monitored as a function of time, temperature, ATP concentration, membrane potential, and phosphorylation state, as well as in the presence of a host of small molecule inhibitors, potentiators, and modifiers. These studies have also added significantly to our knowledge of how ABC transporters function. Nevertheless, expression of CFTR in significant amounts and its subsequent purification has proven to be particularly challenging and success has been limited to a few laboratories10-13.
The need to develop more effective drugs is pressing, yet this process has been hindered by the lack of purified CFTR for screening small molecules. Solving the CFTR expression and purification problem would enable high-throughput drug screening aimed at correcting the primary defect in CF and would also open up a route for high-resolution structural studies to inform rational drug design. Moreover, even relatively basic biochemical characteristics of the protein, such as its functional oligomeric state, interacting proteins and ATPase activity remain poorly characterized. We have previously reported a protocol for the large-scale expression of GFP- and His-tagged murine CFTR in S. cerevisiae14 and now further describe protocols for the purification of CFTR. We have used these methods to purify five orthologues of CFTR, and present data for the purification of chicken CFTR as an example. The selection of detergents for solubilization and purification is a critical step in the purification of any membrane protein. Having screened for the solubility of CFTR in several detergents, we have chosen two contrasting detergents for use in the purification. Dodecyl-β-D-maltoside (DDM) is a nonionic detergent that has been extensively used for both structural and functional studies of membrane proteins15-21. The ionic detergent 1-tetradecanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (LPG-14) is highly efficient in the solubilization of CFTR and has previously been used in the purification of functional membrane proteins10,22,23, including purification of CFTR from S. cerevisiae24.
Protocol
1. Preparation of Buffers
- To make the 100x stock of protease inhibitor (PI) cocktail dissolve 96 mg AEBSF, 3.5 mg chymostatin, 10 mg E64, 16.5 mg leupeptin, 16.5 mg pepstatin, 348 mg PMSF, and 4 mg bestatin in 20 ml DMSO. Make 1 ml aliquots and store at -20 °C. To make a 100x stock of benzamidine, dissolve 720 mg in 20 ml ultrapure water (ddH2O) and store in 1 ml aliquots at -20 °C. This quantity is sufficient for one purification. In all buffers, PI and benzamidine stocks are used at a 1:100 dilution.
- Prepare ‘mPIB’ (0.3 M Tris pH 8, 0.3 M sucrose, 2 mM DTT) and ‘CFTR’ (50 mM Tris pH 8, 20% (v/v) glycerol, 1 mM DTT) buffers and chill to 4 °C. Before use, add 1:100 of the protease inhibitor cocktail and 1:100 benzamidine according to the volume of mPIB used to resuspend the cell pellet (e.g., use 3.5 ml PI and 3.5 ml benzamidine in a total volume of 350 ml mPIB).
- Prepare solubilization buffers. Lyso-phosphatidyl glycerol-14 (LPG) solubilization buffer (50 mM Tris pH 8, 10% (v/v) glycerol, 50 mM NaCl, 1 mM DTT, protease inhibitors (PIs) and 4% (w/v) LPG) and dodecyl maltoside (DDM) solubilization buffer (50 mM Tris pH 8, 20% (v/v) glycerol, 1 M NaCl, 1 mM DTT, protease inhibitors, 4% (w/v) DDM). Buffer can be sonicated in a sonicator bath (35 W, 40 kHz) to assist with dispersal of the detergent, but avoid vortexing the mixture, as this creates bubbles. Chill to 4 °C before use.
- CFTR purification buffer for the LPG purification is 50 mM Tris, 10% (v/v) glycerol, 50 mM NaCl, 1 mM DTT, 0.1% (w/v) LPG-14 and protease inhibitors. Prepare 350 ml of this buffer, and 150 ml of the same buffer plus 1 M imidazole. Adjust pH of both buffers to 8.
- The buffer for purification in DDM consists of 50 mM Tris pH 8, 20% (v/v) glycerol, 1 M NaCl, 1 mM DTT, 0.1% (w/v) DDM. Prepare 350 ml of this buffer, and 150 ml of the same buffer plus 1 M imidazole. Adjust pH of both buffers to 8.
- For gel permeation chromatography (GPC) buffer containing LPG, prepare 50 mM Tris pH 8, 10% (v/v) glycerol, 50 mM NaCl, 1 mM DTT, 0.05% (w/v) LPG-14. For GPC using DDM prepare a buffer of 50 mM Tris pH 8, 20% (v/v) glycerol, 1 M NaCl, 1 mM DTT, 0.1% (w/v) DDM. All buffers and ddH2O used on the GPC column should be filtered (0.2 μm filter) and degassed before use.
- SDS-PAGE sample buffer (2x the working concentration): 50 mM Tris-HCl pH 7.6, 5% (v/v) glycerol, 5 mM EDTA, 0.02% (w/v) bromophenol blue. Make 700 μl aliquots and store at -20 °C. Before use, add 200 μl of 20% (w/v) sodium dodecyl sulfate (SDS) and 100 μl of fresh 0.5 M DTT. Incubate for at least 10 min with sample at room temperature before loading on gel. Do not heat; this will denature the GFP and may cause CFTR to aggregate.
- To make lipid stocks for reconstitution, dissolve a 4:1 (w/w) mixture of E. coli lipids and cholesterol in chloroform and methanol (2:1 v/v), and dry in a glass vial under N2 gas for 2 hr to form a lipid film. Add GPC buffer (with no NaCl) to a lipid concentration of 40 mg/ml and use repeated vortexing and sonication (35 W, 40 kHz) to clarify the solution.
- For the ATPase assay, prepare 100x stocks of ATPase inhibitors by dissolving SCH28080 to 1 mM in DMSO, NaSCN to 1 M in ddH2O and oligomycin to 2.5 mM in 100% (v/v) ethanol. Store in aliquots at -20 °C. Make 100 ml of ATPase buffer with 50 mM Tris pH 7.4, 150 mM NH4Cl, 5 mM MgSO4 and 0.02% (w/v) NaN3. This can be stored at room temperature and used for several assays. Prepare a 5 mM ATP stock immediately prior to use and keep on ice. (N.B. Use Na2ATP to prevent excessive background signal from phosphate in the assay). Prepare the SDS stop solution (12% (w/v) SDS in ddH2O).
- For the Chifflet detection prepare buffer A (3% (w/v) ascorbate, 0.5% (w/v) ammonium molybdate, 0.5 M HCl) immediately before use and buffer B (2% (w/v) sodium citrate, 2% (w/v) sodium meta-arsenite, 2% (v/v) acetic acid).
2. Isolation of Yeast Microsomes
- S. cerevisiae expressing chicken CFTR are grown as described in O’Ryan et al. (2012)14. Store the material from a 20 L fermentation in two aliquots at -80 °C for up to 6 months.
- Defrost one aliquot of cells rapidly and resuspend in 3 ml chilled mPIB per gram of cells.
- Disrupt cells in a bead mill using glass beads of 425-600 μm diameter. Use five 1 min periods of cell disruption separated by 1 min rest periods. (The rest periods are essential to ensure that the cells are not heated during disruption.)
- Monitor cell disruption by centrifugation of a 1 ml sample of the cell lysate from the bead mill. Centrifuge (12,000 x g, 4 °C, 5 min) in a bench top centrifuge. Dilute the supernatant to 1:50 with mPIB in a cuvette and measure the A380. If A380 > 0.1, or has stopped increasing despite several repeated bead-beating cycles, proceed to the following step. If not, repeat 2.3-2.4.
- Centrifuge the total cell lysate (12,000 x g, 4 °C, 20 min). Retain the supernatant. Discard the pellet (containing unbroken cells and mitochondria), but if there is any doubt about the efficiency of cell breakage (see 2.4), then retain the pellet also.
- Centrifuge the supernatant from the previous step (200,000 x g, 4 °C, 1.5 hr). Discard the supernatant and resuspend the pelleted microsomal membranes in CFTR buffer. If the microsomes are intended for purification using DDM, supplement the CFTR buffer with 1 M NaCl.
- Repeat the centrifugation of the resuspended membrane fraction (100,000 x g, 4 °C, 1 hr) and discard the supernatant.
- Resuspend the pelleted microsomes in a minimum volume of CFTR buffer (final volume 5-15 ml, total microsomal protein 70-200 mg). A Bradford assay may be used to determine the total concentration of microsomal proteins25. In addition the fluorescence emission spectrum of the membranes should be measured (excitation = 485 nm, emission = 500-600 nm) and should have a distinct GFP fluorescence peak (maximum at 512 nm). CFTR can be specifically detected on an SDS-PAGE gel, scanned under GFP fluorescence conditions (Figure 1).
- Flash-freeze the resuspended microsomes by plunging into liquid nitrogen and store at -80 °C, or continue to Step 3.
3. Solubilization of Microsomes
- If frozen, defrost microsomes immediately before use in a water bath set to 10 °C.
- For the solubilization of membranes, dilute the microsomes with an equal volume of the relevant solubilization buffer (Step 1.3) to give a final detergent concentration of 2% (w/v) and a microsomal protein concentration 5 mg/ml. Incubate this mixture for 1 hr at 4 °C with agitation (tube rotator). Retain 200 μl for analysis.
- Centrifuge the mixture (100,000 x g, 4 °C, 45 min). Remove the supernatant containing the solubilized membrane proteins, pass it through a 0.45 μm syringe filter and store on ice. Measure the fluorescence of the supernatant (as in Step 2.8).
- Resuspend the insoluble fraction in 1% (w/v) SDS solution to a volume equal to the soluble fraction. Measure the fluorescence in this fraction and retain an aliquot of 50 μl for SDS-PAGE analysis.
4. Nickel-affinity Purification of CFTR
- Link two 5 ml nickel sepharose columns in series. Wash with 2 column volumes (CV) 20% (v/v) ethanol, followed by 2 CV ddH2O, then wash the column with 2 CV of solubilization buffer (Step 1.4-1.5), containing 1 M imidazole. Repeat with 2 CV of solubilization buffer lacking imidazole.
- Add imidazole to a final concentration of 5 mM to the solubilized material (Step 2.8) and manually load the material onto the column or into a sample loop if using an automated liquid chromatography device.
- Load the solubilized material onto the column at a flow rate of 0.5 ml/min, and wash with 2 CV of imidazole-lacking buffer at the same flow rate to remove unbound material. Collect fractions in 50 ml Falcon tubes.
- For the first wash, use 3 CV of purification buffer with 40 mM imidazole at a flow rate of 1 ml/min. Collect 2 ml fractions.
- For the second wash, use 3 CV of purification buffer with 100 mM imidazole. Collect 2 ml fractions.
- Elute CFTR from the HisTrap column with 3 CV of purification buffer with 400 mM imidazole. Collect 2 ml fractions.
- Monitor fluorescence in eluted fractions (Step 2.8).
- Retain aliquots of peak fractions for SDS-PAGE analysis. Flash freeze remaining peak fraction samples and store at -80 °C, or continue to the next purification step.
5. Gel Permeation Chromatography (GPC) Purification of CFTR
- Equilibrate the column (Superose 6 10/300 GL) with 1.2 CV ddH2O followed by 1.2 CV GPC buffer.
- During Step 5.1, concentrate the Ni-affinity purified fractions with the highest GFP fluorescence using a 100,000 MWCO centrifugal filter at 4 °C. If purifying in DDM, avoid concentrating the sample above a protein concentration of 0.3 mg/ml protein as this will cause significant sample loss. Remove the retentate from the concentrator and centrifuge at 100,000 x g for 30 min at 4 °C to pellet large particles.
- Inject this sample onto the column and elute with an isocratic gradient of 1.2 CV GPC buffer. Collect 0.5 ml fractions.
- Measure GFP fluorescence as in section 2.8 to identify those fractions containing CFTR. Retain a small volume (e.g. 50 μl) of each for analysis by SDS-PAGE.
- Freeze fractions in liquid nitrogen and store at -80 °C.
6. Reconstitution of CFTR
- Add lipids (Step 1.8) to the purified CFTR at lipid-to-protein ratio 100:1 (w/w) and incubate at 4 °C for 1 hr. Similarly set up a lipid-only control, substituting the purified protein with the same volume of GPC buffer.
- Remove detergent from the protein/lipid mixture using hydrophobic adsorbent beads. Wash adsorbent beads in 5 CV ddH2O, 5 CV 70% (v/v) ethanol, 5 CV ddH2O, and 5 CV GPC buffer lacking the detergent. Add 200 mg of washed adsorbent beads per ml of purified protein and incubate at 4 °C overnight with gentle agitation.
- Collect the reconstitution sample from the adsorbent beads into a fresh tube using a thin-ended pipette tip.
7. Measurement of ATPase Activity
- Determine the rate of CFTR-specific ATPase activity using a modified Chifflet assay26,27 in a 96-well plate format. With sodium phosphate stock solution (0.65 mM) prepare 0-20 nmol phosphate in a final volume of 50 µl as standards. Use a 1:1 mixture of CFTR buffer and ATPase buffer to dilute the phosphate stock.
- Incubate both reconstituted CFTR and blank liposomes with 1:100 (v/v) ATPase inhibitors (Step 1.9) on ice for 10 min. Use at least 5 μg of reconstituted CFTR.
- Add ATP (Step 1.9) to a final concentration of 2 mM and incubate at 25 °C for 1 hr. Stop the reaction by adding 40 μl of 10% (w/v) SDS (Step 1.9) to each well (including the standards).
- Add 100 μl of buffer A (Step 1.10) and incubate for 10 min. Add 100 μl buffer B (1.10) to each well and measure the absorbance at a wavelength of 800 nm in a 96-well plate-compatible UV/Vis spectrophotometer.
- Convert absorbance at 800 nm into an amount of liberated phosphate using the phosphate standards. Calculate the rate of ATP hydrolysis after subtracting background signal (liposome-only wells).
- For non-reconstituted CFTR follow the same protocol using CFTR buffer for the background readings.
Representative Results
The protocol described above is an efficient means to isolate CFTR-enriched microsomes, with almost complete recovery of CFTR during the cell breakage and preparation of the crude microsomes (Figure 1). Other cell breakage methods may also be employed effectively. We have utilized a French pressure cell, and other high-pressure/cavitation devices (also in combination with impacting against a ruby target) with equal efficiency. For convenience and low initial cost of the equipment, we find the bead-beating method the best.
Using LPG to solubilize and purify CFTR yielded 80 μg protein/L culture at >90% purity (Figure 2). The high yield was due to efficient solubilization of CFTR by LPG (compare Figure 2b, lanes 2 and 4). In addition, efficient and tight binding to the column resulted in minimal loss of CFTR in the unbound fraction and the absence of CFTR in the wash fractions (Figure 2, lanes 3, 5, and 6). The eluted protein had a purity of >90%, estimated by Coomassie-stained SDS-PAGE gels and using densitometry of the CFTR and contaminant bands. Gel permeation chromatography (GPC) separated LPG-purified CFTR from low-molecular weight contaminants (Figure 4, lower panel).
The protocol for CFTR purification using DDM gives purity of about 60% and yield of roughly 50 μg/L (Figure 3). Electron microscopy (EM) of negatively stained fractions from the GPC eluting at about 10 ml (Figure 4) showed that DDM-purified CFTR contains aggregates of 20-30 nm diameter as well as smaller particles of 10 nm diameter (data not shown). It is possible that the small aggregates can reversibly associate and dissociate as ultrafiltration with a 1 MDa cut-off filter failed to remove the EM-detectable aggregates. LPG-purified material did not adsorb to a glow-discharged grid, hence was studied by cryo-EM of unstained fractions. This showed a very homogeneous particle population of a relatively small size (6-8 nm diameter, data not shown).
Finally, the ATPase activity of the purified proteins was measured (Figure 5). As a member of the ABC protein family, CFTR has two nucleotide-binding domains (NBDs) capable of binding and/or hydrolyzing ATP. The data indicate that the purified protein was not able to hydrolyze ATP in the LPG-solubilized state and showed weak ATPase activity in the presence of DDM (Figure 5, unfilled bars). After the addition of lipids, and detergent removal, ATPase activity was 4-fold higher for samples that had been purified in DDM (13 nmol ATP/min/mg protein). The addition of lipids and removal of LPG similarly restored activity to CFTR that had been isolated using LPG, but with a final lower rate (1.5 nmol ATP/min/mg protein) than the DDM-purified and reconstituted material.

Figure 1. Monitoring levels of chicken CFTR in cell lysate (CL), supernatants (S) and pellets (P) during various centrifugation steps used for microsome isolation and washing. SDS-PAGE gels were visualized using the in-gel fluorescence of the GFP tag. The supernatant after cell breakage and centrifugation at 14,000 x g contains virtually all the CFTR (including degradation products). Ultracentrifugation at 200,000 x g sediments all the full-length CFTR leaving some fragments in the supernatant. Ultracentrifugation at 100,000 x g of salt-washed microsomes pellets nearly all the CFTR with the removal of some further fragments.
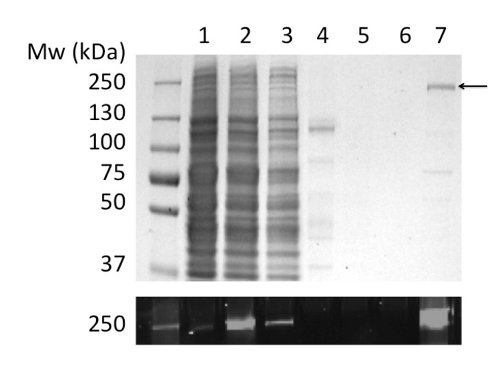
Figure 2. Purification of chicken CFTR in LPG by immobilized metal ion affinity chromatography. Fractions were analyzed by SDS-PAGE followed by Coomassie staining (upper panel) and fluorescence detection of the GFP tag (lower panel). Tracks: (1) Microsomes. (2) LPG-solubilized microsomes. (3) Unbound material. (4) Insoluble material. (5) & (6) 40 and 100 mM imidazole washes. (7) Material eluted with 400 mM imidazole.
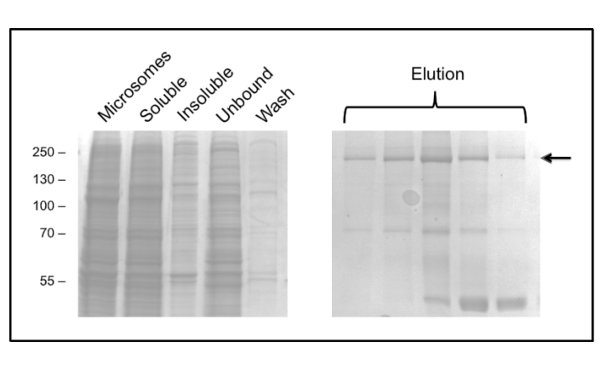
Figure 3. Purification of chicken CFTR in DDM by immobilized metal ion affinity chromatography. Fractions were analyzed by SDS-PAGE followed by Coomassie staining. The left hand panel shows fractions prior to elution. Several consecutive elution fractions are shown in the right hand panel with CFTR indicated by the arrow. Later fractions are enriched in a 40 kDa contaminant, which has been identified by mass spectrometry as ribosomal protein L3.
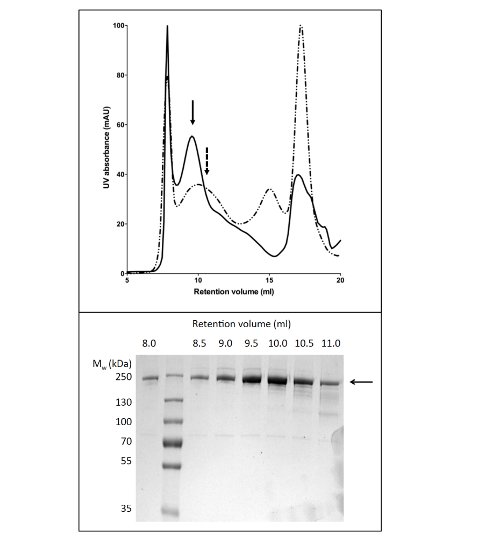
Figure 4. Purification of chicken CFTR by gel permeation chromatography. CFTR purified by Ni-affinity chromatography was concentrated and applied to a GPC column. The elution profile for CFTR (upper panel) purified in buffer containing LPG-14 (solid line) or DDM (dashed line) are overlaid. SDS-PAGE (lower panel) revealed that CFTR eluted between 8 and 11 ml.
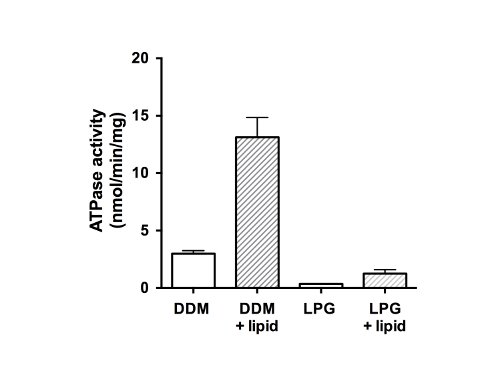
Figure 5. ATPase activity of purified chicken CFTR fractions. Protein purified in DDM or LPG was assayed using a modified Chifflet assay26 in the presence of a cocktail of ATPase inhibitors to eliminate any background ATPase activity from F-, P- and V-type ATPases (unfilled bars). The rate of ATP hydrolysis was also measured after detergent removal and lipid addition (filled bars). The plot shows the mean and standard deviation (n=3). Differences between mean values for ATPase activity in presence and absence of lipid, and difference between activity in DDM and LPG are significant to p<0.05.
Discussion
We have previously described a method for the overexpression of murine CFTR14. Since the publication of that protocol, we have expressed and purified several different orthologs of CFTR using the same system. All orthologs tested so far purified well in the LPG detergent, whilst the DDM purification showed more variation across different orthologs (data not shown). This flexibility illustrates the strength of the yeast approach: it is possible to screen many constructs with relative rapidity in order to select one for a particular purpose.
Washing the yeast microsomes with buffer containing 1 M NaCl prior to solubilization with DDM results in a cleaner microsome preparation and reduces contaminants at later stages. This step is unnecessary in the LPG protocol as the final CFTR sample is >90% pure without the microsome wash. Furthermore, purification in DDM requires several alterations to the buffers for solubilization and purification, namely the addition of extra glycerol and salt. Together, these additions considerably increased the binding of the DDM-solubilized protein to the column.
The DDM purification methodology has scope for improvement, in particular the removal of a 40 kDa major contaminant that, judged by mass spectrometry, is due to the yeast ribosomal subunit L3, which appears to have an inherent affinity for the nickel resin. There is no obvious polyHis sequence in the L3 protein, but examination of its 3D structure when bound to the ribosome (PDB = 1FFK) shows that the folded L3 subunit has a potential polyHis cluster. That this band is less problematic in LPG-purified material may be due to the harsher LPG detergent.
Though the purification in DDM appears to be poorer than that in LPG, milder detergents such as DDM may be more compatible with functional and structural analyses and have already been used in several X-ray crystallographic studies of membrane proteins15-21. Furthermore, our results indicated that the use of LPG leads to loss of ATPase function in CFTR relative to purification in DDM. Hence we would recommend the LPG-based purification protocol for the generation of CFTR where the purity is crucial, for example in applications such as the characterization of post-translational modifications, or in the generation of antibodies, the LPG-based protocol would be chosen. On the other hand in applications where the activity and fully native state of the protein is essential, we would propose the DDM-based protocol as a better option.
To conclude, this protocol describes a reproducible method for the isolation of CFTR in the zwitterionic detergent LPG-14 or the non-ionic detergent DDM. As such it indicates a greater range of purification conditions for CFTR than have previously been reported10-13. In addition milligram quantities of purified CFTR can be obtained using these procedures when combined with a high volume yeast growth system such as a 20 L fermenter and a high capacity cell harvesting system such as a 6 L low speed centrifuge rotor. The CFTR obtained has a cleavable GFP tag which allows easy monitoring of the protein in various biochemical and biophysical assays.
The reagent described in this manuscript (chicken CFTR–containing plasmid or frozen yeast cells) can be obtained through the Cystic Fibrosis Foundation (USA).
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was funded by the US Cystic Fibrosis Foundation (CFF) through its CFTR 3D Structure Consortium. TR was funded by a UK CF Trust studentship, and NC by a UK BBSRC studentship. We acknowledge our colleagues in the CFF CFTR 3D structure consortium for their help and advice and for the design of the codon-optimized chicken CFTR sequence and purification tags.
Materials
| Name of Material/ Equipment | Company | Catalog Number |
| 0.2μM syringe filter | Sartorius | FC121 |
| 100 kDa MWCO centrifugal concentrator (PES membrane) | Vivaspin | VS0641 |
| 2ml microfuge tubes | Sarstedt | 72.695 |
| 40Ti rotor | Beckman Coulter | 337901 |
| 50ml Sterile Falcon Tubes | Sarstedt | 62.547.254 |
| Adenosine triphosphate disodium salt (Na2ATP) | Sigma-Aldrich | A26209 |
| Liquid chromatography system | GE Healthcare | 28-4062-64 |
| Aminoethylbenzenesulfonyl fluoride (AEBSF) | Sigma-Aldrich | A8456 |
| Glass bead-beating cell disrupter | BioSpec | 1107900 |
| Benchtop centrifuge | HERMLE | Z300 |
| Benchtop centrifuge | Eppendorf | 5417R |
| Benchtop microfuge | Fisher | 13-100-511 |
| Benzamidine hydrochloride | Sigma-Aldrich | 434760 |
| Hydrophobic Beads SM-2 Adsorbent | BioRad | 152-3920 |
| Bromophenol blue | Sigma-Aldrich | 114391 |
| Centrifuge tubes | Beckman Coulter | 357000 |
| Gel imaging system | BioRad | 170-808 |
| Cholesterol | Sigma-Aldrich | C8667 |
| Chymostatin | Sigma-Aldrich | C7268 |
| Dimethylsulfoxide (DMSO) | Sigma-Aldrich | D8418 |
| Dithiothreitol (DTT) | Sigma-Aldrich | 43815 |
| E.coli total lipid extract | Avanti lipids | 100500 |
| Epoxysuccinyl-leucylamido-butane (E-64) | Sigma-Aldrich | E3132 |
| Glass beads, acid washed | Sigma | G8772 |
| Glycerol | Fisher | 65017 |
| HisTrap HP columns (5 ml) | GE Healthcare | 17-5247-05 |
| Rapid Coomassie Stain | Novexin | ISB1L |
| Centrifuge JA-17 rotor | Beckman Coulter | 369691 |
| Leupeptin | Merck | 108975 |
| Lyso-phosphatidyl glycerol-14 (LPG) | Avanti lipids | 858120 |
| MgSO4 | Sigma-Aldrich | M7506 |
| Gel tank SDS-PAGE system | BioRad | 165-8006 |
| n-dodecyl-β-D-maltopyranoside (DDM) | Affymetrix | D310S |
| NaCl | Sigma-Aldrich | S6191 |
| NaN3 | Sigma-Aldrich | S2002 |
| NH4Cl | Sigma-Aldrich | A9434 |
| Oligomycin | Sigma-Aldrich | 75351 |
| Ultracentrifuge | Beckman Coulter | 392050 |
| Prestained protein standards | Fermentas | SM1811 |
| Desalting columns (Sephadex G-25) | GE Healthcare | 17-0851-01 |
| Pepstatin A | Sigma-Aldrich | P4265 |
| Phenylmethanesulfonylfluoride (PMSF) | Sigma-Aldrich | P7626 |
| Sch28080 | Sigma-Aldrich | S4443 |
| Sodium dodecyl sulfate (SDS) | Sigma-Aldrich | L37771 |
| Sodium thiocyanate (NaSCN) | Sigma-Aldrich | 251410 |
| Gel filtration 10/300 GL column | GE Healthcare | 17-5172-01 |
| Tris-base | Formedium | TRIS01 |
| Ultracentrifuge tubes | Beckman Coulter | 355618 |
| Vortex mixer | Star Labs | N2400-0001 |
| Ultrasonic water bath | Ultrawave | F0002202 |
| Multimode plate reader | BioTek | BTH1MF |
Referências
- Aleksandrov, A. A., Aleksandrov, L. A., Riordan, J. R. CFTR (ABCC7) is a hydrolyzable-ligand-gated channel. Pflugers Arch. 453, 693-702 (2007).
- Dodge, J. A., Lewis, P. A., Stanton, M., Wilsher, J. Cystic fibrosis mortality and survival in the UK: 1947-2003. EUR RESPIR J. 29, 522-526 (2007).
- O’Sullivan, B. P., Freedman, S. D. Cystic fibrosis. Lancet. 373, 1891-1904 (2009).
- Rommens, J. M., et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 245, 1059-1065 (1989).
- Kariya, C., et al. A role for CFTR in the elevation of glutathione levels in the lung by oral glutathione administration. Am J Physiol Lung Cell Mol Physiol. 292, (2007).
- Gould, N. S., Min, E., Martin, R. J., Day, B. J. CFTR is the primary known apical glutathione transporter involved in cigarette smoke-induced adaptive responses in the lung. Free Radic Biol Med. 52, 1201-1206 (2012).
- Childers, M., Eckel, G., Himmel, A., Caldwell, J. A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates. Med Hypotheses. 68, 101-112 (2007).
- Cole, S. P., et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 258, 1650-1654 (1992).
- Conseil, G., Deeley, R. G., Cole, S. P. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet Genomics. 15, 523-533 (2005).
- Ketchum, C. J., Rajendrakumar, G. V., Maloney, P. C. Characterization of the adenosinetriphosphatase and transport activities of purified cystic fibrosis transmembrane conductance regulator. Bioquímica. 43, 1045-1053 (2004).
- Ramjeesingh, M., et al. A novel procedure for the efficient purification of the cystic fibrosis transmembrane conductance regulator (CFTR). Biochem J. 327 (Pt 1), 17-21 (1997).
- Huang, P., Liu, Q., Scarborough, G. A. Lysophosphatidylglycerol: a novel effective detergent for solubilizing and purifying the cystic fibrosis transmembrane conductance regulator. Anal Biochem. 259, 89-97 (1998).
- Rosenberg, M. F., Kamis, A. B., Aleksandrov, L. A., Ford, R. C., Riordan, J. R. Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem. 279, 39051-39057 (2004).
- O’Ryan, L., Rimington, T., Cant, N., Ford, R. C. Expression and purification of the cystic fibrosis transmembrane conductance regulator protein in Saccharomyces cerevisiae. J Vis Exp. , (2012).
- Oldham, M. L., Chen, J. Snapshots of the maltose transporter during ATP hydrolysis. Proc Natl Acad Sci USA. 108, 15152-15156 (2011).
- Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P., Rees, D. C. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 315, 373-377 (2007).
- Dawson, R. J. P., Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature. 443, 180-185 (2006).
- Gerber, S., Comellas-Bigler, M., Goetz, B. A., Locher, K. P. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 321, 246-250 (2008).
- Ward, A., Reyes, C. L., Yu, J., Roth, C. B., Chang, G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci USA. 104, 19005-19010 (2007).
- Kadaba, N. S., Kaiser, J. T., Johnson, E., Lee, A., Rees, D. C. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science. 321, 250-253 (2008).
- Aller, S. G., et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science. 323, 1718-1722 (2009).
- Koehler, J., et al. Lysophospholipid micelles sustain the stability and catalytic activity of diacylglycerol kinase in the absence of lipids. Bioquímica. 49, 7089-7099 (2010).
- Tian, C., et al. Preparation, functional characterization, and NMR studies of human KCNE1, a voltage-gated potassium channel accessory subunit associated with deafness and long QT syndrome. Bioquímica. 46, 11459-11472 (2007).
- Huang, P., Liu, Q., Scarborough, G. A. Lysophosphatidylglycerol: a novel effective detergent for solubilizing and purifying the cystic fibrosis transmembrane conductance regulator. Anal Biochem. 259, 89-97 (1998).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72, 248-254 (1976).
- Chifflet, S., Torriglia, A., Chiesa, R., Tolosa, S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: Application to lens ATPases. Analytical Biochemistry. 168, 1-4 (1988).
- Rothnie, A., et al. The importance of cholesterol in maintenance of P-glycoprotein activity and its membrane perturbing influence. Eur Biophys J. 30, 430-442 (2001).

