A Research Method For Detecting Transient Myocardial Ischemia In Patients With Suspected Acute Coronary Syndrome Using Continuous ST-segment Analysis
Summary
Continuous 12-lead electrocardiographic (ECG) monitoring can identify transient myocardial ischemia, even when asymptomatic, among patients with suspected acute coronary syndrome (ACS). In this article we describe our method for initiating patient monitoring using a Holter device, downloading the ECG data for off-line analysis, and how to utilize the ECG software to identify transient ischemia.
Abstract
Each year, an estimated 785,000 Americans will have a new coronary attack, or acute coronary syndrome (ACS). The pathophysiology of ACS involves rupture of an atherosclerotic plaque; hence, treatment is aimed at plaque stabilization in order to prevent cellular death. However, there is considerable debate among clinicians, about which treatment pathway is best: early invasive using percutaneous coronary intervention (PCI/stent) when indicated or a conservative approach (i.e., medication only with PCI/stent if recurrent symptoms occur).
There are three types of ACS: ST elevation myocardial infarction (STEMI), non-ST elevation MI (NSTEMI), and unstable angina (UA). Among the three types, NSTEMI/UA is nearly four times as common as STEMI. Treatment decisions for NSTEMI/UA are based largely on symptoms and resting or exercise electrocardiograms (ECG). However, because of the dynamic and unpredictable nature of the atherosclerotic plaque, these methods often under detect myocardial ischemia because symptoms are unreliable, and/or continuous ECG monitoring was not utilized.
Continuous 12-lead ECG monitoring, which is both inexpensive and non-invasive, can identify transient episodes of myocardial ischemia, a precursor to MI, even when asymptomatic. However, continuous 12-lead ECG monitoring is not usual hospital practice; rather, only two leads are typically monitored. Information obtained with 12-lead ECG monitoring might provide useful information for deciding the best ACS treatment.
Purpose. Therefore, using 12-lead ECG monitoring, the COMPARE Study (electroCardiographic evaluatiOn of ischeMia comParing invAsive to phaRmacological trEatment) was designed to assess the frequency and clinical consequences of transient myocardial ischemia, in patients with NSTEMI/UA treated with either early invasive PCI/stent or those managed conservatively (medications or PCI/stent following recurrent symptoms). The purpose of this manuscript is to describe the methodology used in the COMPARE Study.
Method. Permission to proceed with this study was obtained from the Institutional Review Board of the hospital and the university. Research nurses identify hospitalized patients from the emergency department and telemetry unit with suspected ACS. Once consented, a 12-lead ECG Holter monitor is applied, and remains in place during the patient’s entire hospital stay. Patients are also maintained on the routine bedside ECG monitoring system per hospital protocol. Off-line ECG analysis is done using sophisticated software and careful human oversight.
Introduction
According to the most recent American Heart Association statistics, coronary artery disease (CAD) was estimated to be responsible for 1.2 million hospital stays and was the most expensive medical condition treated.1 More than half of the hospital stays for CAD were among patients who also received percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) during their stay. Because of the prevalence of CAD, clinicians working in hospitals are likely to encounter these patients frequently. The purpose of this manuscript is to describe the ECG application and analysis methodology used in the COMPARE Study (electroCardiographic evaluatiOn of ischeMia comParing invAsive to phaRmacological trEatment; (R21 NR-011202), which might be utilized by other researches exploring this clinical problem.
Each year, nearly 700,000 Americans will have a new coronary attack, or acute coronary syndrome (ACS).1 The pathophysiology of ACS involves rupture of an atherosclerotic plaque; hence, treatment is aimed at plaque stabilization in order to prevent cellular death. However, there is considerable debate among clinicians, about which treatment pathway is best: early invasive using percutaneous coronary intervention (PCI/stent) when indicated or a conservative approach (i.e., medications only or PCI/stent if recurrent symptoms occur).2-5
There are three types of ACS: ST elevation myocardial infarction (STEMI), non-ST elevation MI (NSTEMI), and unstable angina (UA).6 Among the three types, NSTEMI/UA is nearly four times as common as STEMI.7,8 Treatment decisions for NSTEMI/UA are based largely on symptoms and resting or exercise electrocardiograms (ECG). However, because of the dynamic and unpredictable nature of the atherosclerotic plaque, these methods often under-detect myocardial ischemia because symptoms are unreliable, and/or continuous ECG monitoring was not utilized.
The manifestations of NSTEMI/UA may include (1) symptoms (chest, arm, jaw or neck pain, sweating, nausea), (2) release of cardiac biomarkers in the case of NSTEMI (Troponin I, Troponin T, or creatnine kinase-MB) and (3) electrocardiographic (ECG) changes (intermittent ST elevation/depression, or T-wave inversion). While symptoms may occur in ACS, it is well documented that transient episodes of ECG-detected ischemia are clinically silent in over 70% of patients.9-12 Importantly, many studies have found that patients with ECG-detected ischemia, as compared to patients without such events, were at higher risk for unfavorable outcomes in both the short term11-13 and long term.14-16 Hence, symptoms are an unreliable indicator of ischemia in NSTEMI/UA, which is problematic since treatment decisions for an early invasive strategy or initial medication strategy are often driven by patient symptoms.2-5 Further, biomarkers identify NSTEMI/UA patients too late because their presence in the serum indicates that cellular death has already occurred.
Electrocardiographic changes indicative of ischemia occur within seconds of diminished coronary blood flow.10,17 The portion of the ECG complex that changes during acute ischemia is the ST-segment, which is measured at one of three points; (1) J-point, (2) J point plus 60 milliseconds (msec) or, (3) J-point plus 80 (msec) (Figure 1).
The 12-lead ECG has important advantages over both symptoms and biomarkers in identifying MI in that it is non-invasive, inexpensive, and if maintained continuously can identify transient ischemia, even when it is clinically silent. While 12-lead ECG monitoring is ideal because multiple areas of the heart are assessed, typical hospital practice incorporates monitoring with only two ECG leads. The two ECG leads most commonly monitored by nurses in the hospital setting are leads V1 and II.18 Unfortunately, these two leads are not sensitive for detecting transient ischemia in many ACS patients.10,19 Therefore, ischemia occurring outside of these two monitored leads will be missed.
Using 12-lead ECG monitoring the COMPARE Study is designed to assess the frequency and clinical consequences of transient myocardial ischemia, in patients with NSTEMI/UA treated with early invasive PCI/stent compared to those managed with an initial conservative approach, which includes medications alone, with transition to PCI/stent following recurrent symptoms. In our study, continuous 12-lead ECG Holter recorder is used to capture ischemia. The aim of this descriptive study is to assess the frequency and clinical consequences of transient myocardial ischemia, in patients with NSTEMI/UA treated with either early invasive PCI/stent or those managed conservatively (medications or PCI/stent following recurrent symptoms).
Methodological Challenges
While computer-assisted ST-segment software works well for detecting transient ischemia, accurate analysis requires careful, expert human oversight. Important factors to consider during analysis include (1) artifact, (2) the consistency and accuracy electrode placement, (3) body position changes, (4) drug effects, and (5) sudden waveform changes.20 Each of these factors will be discussed below.
Artifact: Because clinically significant ST-segment changes are as small as 100 microvolt (one small box on the ECG paper), noisy signal from muscle artifact can significantly hinder analysis. Most artifacts are related to improper skin preparation.21 The problem can be easily solved with careful skin preparation of the patient’s torso at the outset of monitoring and as needed throughout the monitoring period.21-23 Skin preparation should include removal of hair at the sites of electrodes, vigorous removal of skin oil/debris, and use of an alcohol prep pad and/or a washcloth.23,24
Consistency and Accuracy of Electrode Placement: False positive ST-segment changes can occur when electrodes are moved or removed during monitoring.20 This can be managed by marking each electrode site with indelible ink at the outset of monitoring to ensure that electrodes are reapplied to the correct location if they fall off or are removed for procedures (e.g., echocardiogram, X-rays).25 We recommend an assessment of the patient’s torso at least every 4 hr to ensure skin electrodes are on the chest.
Body Position Changes: Studies using continuous ST-segment monitoring have shown that some patients can exhibit concomitant QRS and ST-segment amplitude changes during body position changes (i.e., left-, or right-side lying), which can be mistaken for myocardial ischemia.26,27 Our research team found that body position changes were the most common cause of false positive ST-segment alarms.20 Using echocardiography, Feldman and co-workers28 found that when patients moved from a supine to a left side-lying position, the left ventricle moved closer to the lateral chest wall. This had a direct effect on the distance of the left ventricle from the chest electrodes and resulted in an increase in the amplitude of the R-wave in the ECG leads over this myocardial territory. These changes have been confirmed by others.26,27 In general, positional ECG changes are suspected when ECG waveform amplitudes increase with concomitant ST-segment changes. A helpful strategy is to obtain ‘positional template ECGs’ with patients assuming supine, left- and right-side lying positions when monitoring is initiated, for later comparison during the analysis.
Pharmacological Effects: Even in therapeutic doses drugs can affect ECG waveforms. Drugs that alter the ST-segment are particularly important since they lead to a misdiagnosis of myocardial ischemia.29 These drugs include digitalis, antiarrhythmics, and phenothiazines. The “digitalis effect” is used to describe the characteristic ST-segment and T wave changes that can occur with this drug, including ‘coved’ ST-segment depression, a flattened T-wave and a short QTc interval.30 Other drugs, such as the antiarrhythmics (i.e., quinidine, sotalol and amiodarone)30 and phenothiazine, used to treat schizophrenia/psychotic disorders, increase the QTc interval, and decrease T-wave amplitude.31
Nevertheless, patients taking these drugs can be monitored for acute ST-segment changes that may indicate acute myocardial ischemia. The baseline abnormalities seen with the drugs are chronic; hence any ST-segment deviation (depression or elevation) that occurs from the patient’s baseline ST-segment level should be assessed for acute ischemia.
Sudden Waveform Changes: Transient conditions, such as arrhythmias, right- or left bundle branch block (BBB), and intermittent ventricular paced beats, can distort the ST-segment and lead to a false-positive ischemia diagnosis.20
In summary, continuous ST-segment monitoring is an excellent tool for identifying transient myocardial ischemia in patients with suspected ACS. However, this method requires that careful application of electrodes and leads wires is performed at the initiation and throughout monitoring. This method also requires careful human oversight in order to eliminate false positive ST-segment changes.
Step-by-Step Methodology
In the COMPARE study, a digital Holter recorder is used (H12+, Mortara Instruments, Milwaukee, WI), which records electrical potentials sampled at 4-msec intervals over a 10-sec period to identify a median beat. All 12 ECG leads (I, II, III, aVR, aVL, aVF, V1-V6) are simultaneously acquired at a digital sampling rate of 1,000 samples per sec per channel which is stored to a compact flash memory card. In addition, the H12+ features electrode impedance measurement to ensure proper skin preparation prior to beginning the Holter recording. A display indicates if an ECG lead has become detached and also provides an internal clock for recording of diary events. Data from the Holter monitor flashcard is then downloaded to the H-Scribe, an off-line Holter Analysis System (Mortara Instruments, Milwaukee, WI). The software allows analysis of continuous ECG recordings to determine the quality of the signal, presence of arrhythmias, and ischemia.
Research assistants with experience working with the cardiac patient population collect the ECG Holter data. A training session at the start of the study was scheduled to ensure the quality and consistency of the study protocol. A training session covering the ECG monitoring procedure, including how to apply ECG monitoring equipment, initiate/maintain ECG monitoring, and download ECG Holter data onto the research computer was done. Return demonstrations were performed and inter-rater reliability is done throughout the study by randomly assessing 10% of currently enrolled subjects. Bi-weekly research team meetings ensure that recruitment goals are being met, challenges are discussed, and results of inter-rater reliability tests can be shared.
The research methodology used in the COMPARE Study has two important steps; patient preparation and off-line ECG analysis. Both of these methods will be explained in a step-by-step manner in the subsequent section.
Protocol
1. Patient Preparation
- Obtaining informed consent.
- Identify land marks on the chest for accurate lead placement. In order to minimize ECG artifact and noise the Mason-Likar electrode configuration is used in which limb lead electrodes are placed on the torso rather than the distal extremities (Figure 2).23
- Prep the skin of the torso with alcohol prep pads and dry briskly with gauze pads to ensure optimal skin electrode contact.
- If necessary, clip chest hair at the specific torso locations in order to make adequate contact with the skin electrode. Perform this step with caution as many of this patient population receive anticoagulation therapy and are therefore prone to bleeding.
- Use indelible ink, if possible, to mark the skin electrode sites to ensure electrodes are returned to the correct location in the event they fall off or are removed for procedures (i.e., echocardiograms, chest X-ray, etc.).
- In women, electrode V3 should be placed on top of the breast tissue and electrodes V4 and V5 should be placed immediately below a pendulous breast so that the breast lies on top of the electrode in order to ensure accurate placement and prevent motion artifact.
- Dual monitoring is used in this study, the hospital bedside monitor and the research Holter recorder. Hence, two ECG skin electrodes are applied to the torso.
- Connect ECG lead wires to the skin electrodes before placing the electrodes on the patient’s chest to prevent unnecessary pressure on the chest.Radiolucent skin electrodes are applied in order to avoid removal for radiographic procedures.
- Enter the patient’s unique research identification number (de-identified) into the Holter device and start ECG monitoring.
- Place the Holter recorder in a monitor pouch, and place via a lanyard around neck or place in the gown pocket.
- Obtain positional ECGs for use during the off-line analysis of the ECG data. The three positions to include are: 1) supine, 2) right side, and 3) left side lying.
- Assess the patient at least every 2 hr when the research staff is present in the hospital and at the end of the day-time shift to ensure monitoring is maintained over night. Staff education of nurses, and ancillary staff (i.e., X-ray, echocardiography, and catheterization technicians) is done to ensure skin electrodes are replaced if removed for a procedure. A torso diagram with correct electrode placement is left at the bedside as a resource so that staff can replace the skin electrodes if necessary as well as extra skin electrodes.
- If the patient goes to the cardiac catheterization laboratory, connect the radiolucent lead wires to the skin electrodes so that ECG monitoring can be maintained during the catheterization procedure to ensure continuous monitoring.
- When monitoring is completed, the equipment is cleaned with antimicrobial wipes.
2. ECG Analysis Procedure
- At the completion of monitoring (may vary in duration), load the compact flashcard from the ECG Holter recorder onto the flashcard reader of H-Scribe research computer.
- Prepare the data for analysis by identifying and labeling each QRS complex as normal, ventricular, supraventricular or artifact with the H-Scribe arrhythmia analysis software.
- Initial assessment of the ECG data is done without knowledge of clinical data, to minimize bias.
- The H-Scribe software is programmed to identify transient myocardial ischemia using the standard definition; ST deviation (elevation or depression) ≥ 100 microvolts in ≥ 2 ECG lead(s) lasting ≥ 60 sec.32 In this study, ST-segment deviation is measured at 60 msec past the J-point.
- ST-segment changes meeting these criteria are identified and labeled by the H-Scribe and indicated on the analysis summary. Human oversight to verify whether ischemia is present must be done for any computer generated event in order to ensure false positive ST-segment changes are identified correctly. This process will be described below.
- ST-segment trends are assessed for changes suggestive of acute ischemia an efficient method of evaluating hours of continuously acquired ECG data (Figure 3).
- In order to identify true myocardial ischemia, any trend suggestive of acute ischemia is assessed by obtaining three ECGs:
- Baseline ECG prior to the ST-segment changes, identified as ‘pre-event ECG’
- An ECG during the maximum point of ST segment deviation, identified as ‘max event ECG’
- An ECG when ST-segment deviation has completely resolved, identified as ‘post-event ECG’
- Assess each of the three ECGs to ensure the ST changes are not due to false positive changes (e.g., accelerated ventricular rhythm, intermittent ventricular pacing rhythm, intermittent bundle branch block, or body position changes).20
- Missing ECG data, data of insufficient quality and false-positive ST changes are excluded from the analysis. These problems will be minimized by using several strategies. These include (1) use of radiolucent ECG electrodes, eliminating the need to remove the electrodes for X-rays; (2) high quality skin prep by research nurses performed at the outset of monitoring; (3) rounding every 2 hr on patients throughout the day; and (4) availability by phone to answer concerns or questions from the nursing and medical staff. Additionally, the research team communicates with both nursing and medical staff on a regular basis.
- Once the ECG data is analyzed the patient’s medical record is used to identify dates/times of treatments, medications and symptoms during the monitoring period.
- Verification of ECG analysis is ensured by having a second expert analyze the ECG data using the same methodology described above in a designated proportion of the sample. Our team does this for 20% of the patients enrolled.
- For research purposes, archive de-identified ECG data onto an external hard drive and/or onto a network drive to ensure the data is saved appropriately.
Representative Results
The initial analysis of the ECG data includes an assessment of the ST-segment trends in each of the 11 leads using the H-Scribe software. Of note, lead aVR is not displayed on trend because it’s an inverted lead and is not considered useful for trend analysis; however, this lead is visible on the 12-lead ECG printout. This method is a quick and easy assessment used to evaluate hours of ECG data for the presence of possible transient myocardial ischemia. A change in the ST-segment trend requires further in-depth analysis of printed 12-lead ECG’s before, during and after the trend change and will be explained below.
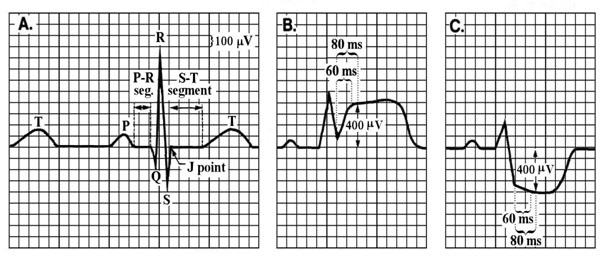
Figure 1. Panel A shows a normal ST-segment, which is flat compared to the PR segment; B showsST-segment elevation, typically indicating total coronary occlusion; and C shows ST-segment depression, typically indicating partial coronary occlusion. ST-segment deviation can be measured at the J-point, J-point + 60 msec, or J-point + 80 msec. (Source: Pelter, M. & Carey, M. in AACN Procedure Manual for Critical Care (ed Wiegand Lynn McHale DJ) 511-518 (Elsevier Saunders, 2011 with permission).
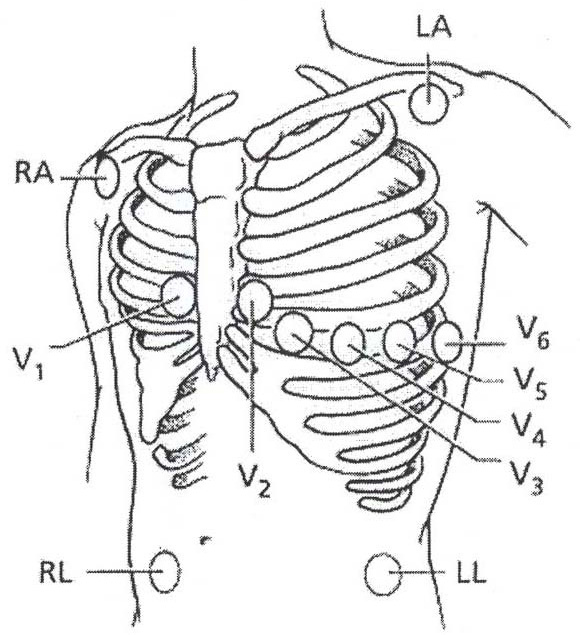
Figure 2. Torso illustrates lead locations used for the Mason-Likar electrode configuration. Right Arm (RA) Infraclavicular fossa close to the right shoulder; Left Arm (LA) infraclavicular fossa close to the left shoulder; Right Leg (RL) Lower right abdomen below the umbilicus; Left Leg (LL) lower left abdomen below the umbilicus; V1 4th intercostal space right of the sternal boarder; V2 4th intercostal space left of the sternal boarder; V3 Mid way between V2 and V4; V4 5th intercostal space midclavicular line; V5 Straight line from V4 anterior axillary line; V6 Straight line from V4 midaxillary. (Source: Pelter, M. & Carey, M. in AACN Procedure Manual for Critical Care (ed Wiegand Lynn McHale DJ) 511-518 (Elsevier Saunders, 2011 with permission).
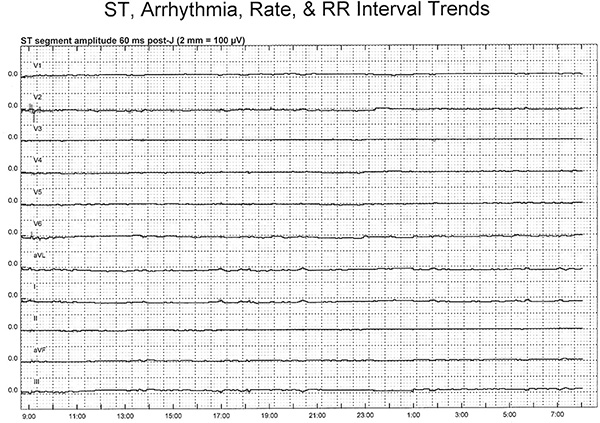
Figure 3. Illustrates a ‘normal,’ or non-ischemic ST-segment trend in a patient with 23 ½ hr of ST-segment monitoring. On the Y-axis, are the 11 ECG leads, with lead V1 shown at the top and lead III at the bottom. The 0.0 on the Y-axis indicates a normal or isoelectric ST-segment. The X-axis displays a 24 hr time period. In this example, ECG monitoring was initiated just before 0900 and ended at 0800 the next morning. The ST-segments in this patient remain at the 0.0 point throughout the monitoring period (solid line), indicating there were no ST-segment shifts indicative of transient myocardial ischemia. A 12-lead ECG can be obtained from any point in time during the monitoring period by simply selecting a specific time and printing the ECG. It is possible to save any ECG(s) in a report format for the research documentation file. Click here to view larger figure.
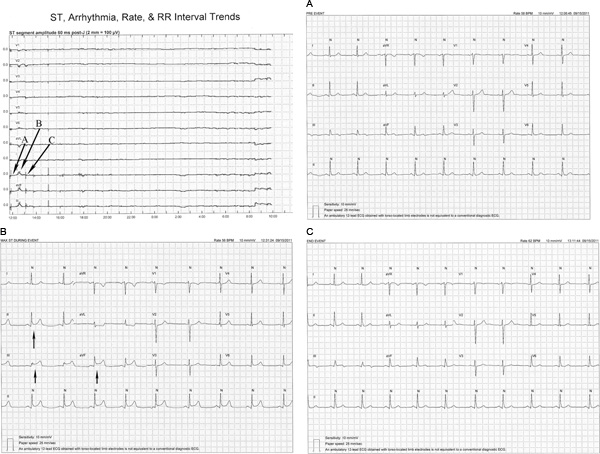
Figure 4. In this example, a transient ST-segment elevation event is illustrated. Monitoring is initiated just before 1200 and is maintained continuously until 1000 the next day (X-axis). At 1230, less than 1 hr after monitoring was initiated, ST-segment elevation is seen in leads II, aVF and III (see bottom three ST-segment trends). 4A, 4B, and 4C are 12-lead ECG’s printed pre-event, during maximal ST-segment changes, and post-event and show abrupt ST-segment elevation in lead II, aVF, and III suggestive of complete coronary occlusion, that resolves after 1 hr. Of note, this patient was asymptomatic and the ST segment changes were not detected by the hospital staff. The ST-segment trend also shows two additional small but brief ST-segment changes in these same leads at 1500 and just before 1800. Because these ST-segment changes did not exceed the 100 microvolt threshold required for an ST event they were not counted as transient ischemic events. The following morning at 0800 this patients was taken to the cardiac catheterization laboratory based on his symptoms and troponin (2.5 μg/L). A 90% lesion was found in the right coronary artery and a stent was placed. The ST-segment elevation at the end of the monitoring period occurred during the percutaneous coronary intervention procedure including stent placement and resolved following the procedure. 4A. Pre-event transient ischemic event ECG. 4B. Maximal ST elevation during ischemic event. Note ST-segment elevation of 200 microvolts in leads II, III, and aVF. 4C. 12-lead ECG following the ischemic event. Note that ST-segment elevation has resolved in leads II, III, and aVF (arrows). Click here to view larger figure.
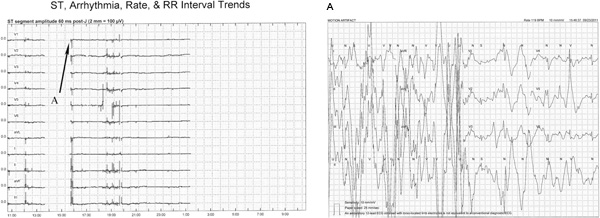
Figure 5. This figure illustrates two issues that can occur during Holter monitoring, interruption of continuous monitoring due to; (1) detached lead wires or skin electrodes, and (2) motion artifact. In both cases, ECG data is not available for analysis. Just prior to 1400, the ST-segment trend disappears in all of the leads when the right leg electrode (ground electrode) became detached. When the lead was replaced at 1600 monitoring resumed. Unfortunately, this same problem occurs just after 0100 and continued until the end of the recording. Click here to view larger figure.
Also illustrated in this figure when monitoring resumes just prior to 1600 is an abrupt change in the ST-trend. A similar pattern of irregular ST-segment trend changes are seen at approximately 1200, and from 1830 to 2000. 5A shows motion artifact as the sources of the ST-segment changes, and was also the source of the other irregular trend changes. In general, abrupt and or erratic changes of the ST-segment trend often indicate motion artifact and or body position changes, rather than myocardial ischemia. However, ECGs from before, during and after ST-segment trend changes should be printed and carefully evaluated for possible ischemia.
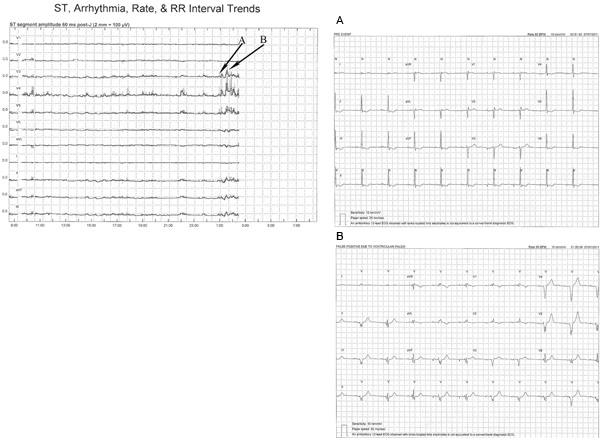
Figure 6. This ST-segment trend show false positive ST-segment changes due to intermittent ventricular pacer. Just prior to 0100 the ST-segment trend changes showing ST-segment elevation in lead V3, V4, V5, II, aVF and III. 6A is a 12-lead ECG obtained at 00:51:02 just prior to the trend change. Of note, are atrial pacer spikes visible in the last 4 beats of the 10 sec rhythm strip in lead II (see bottom of figure). Note ST-segment depression in leads II, III, aVF, V5 and V6 likely due to the drug digitalis, which this patient was taking. These changes are chronic and were present throughout the monitoring period. 6B shows an ECG obtained at 01:20:39 where ventricular pacing is now present, atrial pacing is visible as well. The result of ventricular pacing is a change of the ST segment from predominantly negative to predominately positive in leads V3, V4, V5, II, aVF, resulting in ST-segment elevation. However, these changes are not due to ischemia but rather abnormal depolarization and repolarization associated with ventricular pacer. Click here to view larger figure.
Discussion
In this article a research methodology using Holter recordings that capture continuous 12-lead ECG recordings is described. While computer-assisted ST-segment software works well for detecting transient ischemia, accurate analysis requires careful, expert human oversight. Important factors to consider during analysis include (1) artifact, (2) the consistency and accuracy electrode placement, (3) body position changes, (4) drug effects, and (5) sudden waveform changes.20
In summary, continuous 12-lead ECG monitoring, which is both inexpensive and non-invasive, can identify transient episodes of myocardial ischemia, a precursor to MI, even when asymptomatic.10,12,33,34 However, 12-lead ECG monitoring is not usual hospital practice; rather, only two leads are typically monitored. Information obtained with 12-lead ECG monitoring might provide useful information for deciding on the best treatment in patients with ACS. However, randomized clinical trials assessing clinician responses to ischemia are needed to determine the value of this technology for identifying high risk patients that might benefit from more aggressive management strategies during ACS.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was funded by a grant from the National Institutes for Nursing Research R21 NR-011202 (MMP); R21 NR-009716 (MGC).
The authors would like to thank Debbie Ganchan, RN, BSN for her careful and thoughtful data collection. We would also like to thank Saint Mary’s Hospital as well as the nurses and physicians at the hospital who have generously offered their assistance with the study.
Materials
| Name of Reagent/Material | Company | Catalogue Number | Comments |
| Skin Electrodes: Bio ProTech Foam Radiotranslucent Electrode | Cardiac Direct | SKU: T716C | |
| Radiolucent Leadwires | Advantage Medical Cables | LW-3090R48/5A | |
| Holter Recorder: H12+ 12-lead Holter recorder, Version 3.12, 24 hr Compact Flash card, American Heart Association 10 wire LeadForm patient cable | Mortara Instrument, Inc. Milwaukee, WI | H12PLUS-AAA-XXXXX | |
| H-Scribe ECG Holter Analysis System, Version 3.71 | Mortara Instrument, Inc. Milwaukee, WI | HSCRIBE – BAA – AACXX |
Referências
- American Heart Association. . Heart Disease and Stroke Statistics Update. , (2012).
- Bavry, A. A., Kumbhani, D. J., Rassi, A. N., Bhatt, D. L., Askari, A. T. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J. Am. Coll. Cardil. 48, 1319-1325 (2006).
- de Winter, R. J., et al. Early invasive versus selectively invasive management for acute coronary syndromes. N. Engl. J. Med. 353, 1095-1104 (2005).
- Hirsch, A., et al. Long-term outcome after an early invasive versus selective invasive treatment strategy in patients with non-ST-elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow-up study. Lancet. 369, 827-835 (2007).
- Mehta, S. R., et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. Jama. 293, 2908-2917 (2005).
- Rosamond, W., et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 115, e69-e171 (2007).
- dos Santos, E. S., S, E., et al. Acute coronary syndrome registry at a cardiology emergency center. Arq Bras Cardiol. 87, 597-602 (2006).
- Thom, T., et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 113, e85-e151 (2006).
- Adams, M. G., Pelter, M. M., Wung, S. F., Taylor, C. A., Drew, B. J. Frequency of silent myocardial ischemia with 12-lead ST segment monitoring in the coronary care unit: are there sex-related differences?. Heart Lung. 28, 81-86 (1999).
- Drew, B. J., et al. 12-lead ST-segment monitoring vs single-lead maximum ST-segment monitoring for detecting ongoing ischemia in patients with unstable coronary syndromes. Am. J. Crit Care. 7, 355-363 (1998).
- Gottlieb, S. O., Weisfeldt, M. L., Ouyang, P., Mellits, E. D., Gerstenblith, G. Silent ischemia as a marker for early unfavorable outcomes in patients with unstable angina. N. Engl. J. Med. 314, 1214-1219 (1986).
- Pelter, M. M., Adams, M. G., Drew, B. J. Transient myocardial ischemia is an independent predictor of adverse in-hospital outcomes in patients with acute coronary syndromes treated in the telemetry unit. Heart Lung. 32, 71-78 (2003).
- Goodman, S. G., et al. Randomized trial of low molecular weight heparin (enoxaparin) versus unfractionated heparin for unstable coronary artery disease: one-year results of the ESSENCE Study. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q Wave Coronary Events. J. Am. Coll. Cardiol. 36, 693-698 (2000).
- Amanullah, A. M., Lindvall, K. Prevalence and significance of transient–predominantly asymptomatic–myocardial ischemia on Holter monitoring in unstable angina pectoris, and correlation with exercise test and thallium-201 myocardial perfusion imaging. Am. J. Cardiol. 72, 144-148 (1993).
- Betriu, A., et al. Recurrent ischemia after thrombolysis: importance of associated clinical findings. GUSTO-I Investigators. Global Utilization of Streptokinase and t-PA [tissue-plasminogen activator] for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 31, 94-102 (1998).
- Bugiardini, R., et al. Relation of severity of symptoms to transient myocardial ischemia and prognosis in unstable angina. J. Am. Coll. Cardiol. 25, 597-604 (1995).
- Krucoff, M. W. Electrocardiographic monitoring and coronary occlusion. Fingerprint pattern analysis in dimensions of space, time, and mind. J. Electrocardiol. , 232-237 (1989).
- Drew, B. J., Ide, B., Sparacino, P. S. Accuracy of bedside electrocardiographic monitoring: a report on current practices of critical care nurses. Heart Lung. 20, 597-607 (1991).
- Drew, B. J., Adams, M. G., Pelter, M. M., Wung, S. F. ST segment monitoring with a derived 12-lead electrocardiogram is superior to routine cardiac care unit monitoring. Am. J. Crit. Care. 5, 198-206 (1996).
- Drew, B. J., Wung, S. F., Adams, M. G., Pelter, M. M. Bedside diagnosis of myocardial ischemia with ST-segment monitoring technology: measurement issues for real-time clinical decision making and trial designs. J. Electrocardiol. 30, 157-165 (1998).
- Clochesy, J. M., Cifani, L., Howe, K. Electrode site preparation techniques: a follow-up study. Heart Lung. 20, 27-30 (1991).
- Medina, V., Clochesy, J. M., Omery, A. Comparison of electrode site preparation techniques. Heart Lung. 18, 456-460 (1989).
- Pelter, M., Carey, M., Wiegand Lynn McHale, D. J. . AACN Procedure Manual for Critical Care. , 511-518 (2011).
- Pelter, M. M. Electrocardiographic monitoring in the medical-surgical setting: clinical implications, basis, lead configurations, and nursing implications. Medsurg Nurs. 17, 421-428 (2008).
- Carey, M., Pelter, M., Carlson, K. K., Wiegand Lynn McHale, D. J. . AACN Procedure Manual for Critical Care. , 430-437 (2010).
- Adams, M. G., Drew, B. J. Body position effects on the ECG: implication for ischemia monitoring. J. Electrocardiol. , 285-291 (1997).
- Adams, M. G., Drew, B. J. Efficacy of 2 strategies to detect body position ST-segment changes during continuous 12-lead electrocardiographic monitoring. J. Electrocardiol. 35, 193-200 (2002).
- Feldman, T., Borow, K. M., Neumann, A., Lang, R. M., Childers, R. W. Relation of electrocardiographic R-wave amplitude to changes in left ventricular chamber size and position in normal subjects. Am J Cardiol. 55, 1168-1174 (1985).
- Carey, M. G., Ponivas, S. J., Pelter, M. M. Differentiating ST-Segment Strain Pattern From Acute Ischemia. Am. J. Crit. Care. 15, 321-322 (2006).
- Wagner, G. S. . Marriott’s Practical Electrocardiography. , 232-238 (2008).
- Timour, Q., et al. Sudden death of cardiac origin and psychotropic drugs. Front Pharmacol. 3, 76 (2012).
- Drew, B. J., et al. AHA scientific statement: practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association Scientific Statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized electrocardiology and the American Association of Critical-Care Nurses. J. Cardiovasc. Nurs. 20, 76-106 (2005).
- Drew, B. J., et al. Frequency, duration, magnitude, and consequences of myocardial ischemia during intracoronary ultrasonography. Am. Heart J. 134, 474-478 (1997).
- Drew, B. J., Pelter, M. M., Adams, M. G. Frequency, characteristics, and clinical significance of transient ST segment elevation in patients with acute coronary syndromes. Eur. Heart J. 23, 941-947 (2002).

