Establishing a Primary Dorsal Root Ganglia Cell Culture from a Rat Spinal Column
Abstract
Source: Lin, Y., et al. Dorsal Root Ganglia Isolation and Primary Culture to Study Neurotransmitter Release. J. Vis. Exp. (2018).
This video demonstrates a technique for isolating and culturing dorsal root ganglia (DRG) cells from the rat spinal column. DRGs excised from the spinal column are treated with enzymes and mechanically dissociated to obtain a single-cell suspension. The cells are cultured in a medium containing inhibitors and growth factors that limit glial cell proliferation and promote neuronal growth, respectively.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Collect Lumbar DRG from Experimental Rats
- Use 2- to 3-week-old Sprague-Dawley (SD) rats for lumbar DRG collection.
NOTE: DRG neurons collected from rats over 4 weeks of age do not grow well under the culture conditions described herein. - Sterilize all surgical instruments in an autoclave.
- Anesthetize the rat with a 1:1 mixture of tiletamine and zolazepam (20 mg/kg; intraperitoneal injection (IP)) and wait until the animal shows no foot-withdrawal response in a toe-pinch test.
NOTE: Different anesthesia strategies can be used successfully in this protocol. - Sacrifice the rat by decapitation with a commercial guillotine.
- Use the guillotine to isolate the body trunk of the rat between the forelimb and femur. See Figure 1A for a diagram of the region to be collected.
NOTE: The caudal cut line should be just rostral to the femur. The lumbar L6 DRG will be excised if the cut site is too high in the spinal column. - Cut along the sternum and remove all organs/tissues with dissection scissors (Figure 2A-a).
- Cut along the side of trunk to collect the dorsal part of the rat and remove the skin. See Figure 1B for a photograph of the dissected dorsal trunk.
- Prepare the tissue on ice before collecting DRG. Clean the fur and blood from gloves and sterilize them with 75% ethanol before proceeding to the next step.
- Remove the muscles covering the lumbar spine. First, make two cuts along the sides of the spinal column (left and right) and one lateral cut to mark the rostral extent of the lumbar spine. Then, remove the dorsal muscles of the spine with bone cutting forceps (Figure 2A-b).
- Remove the dorsal portion of the vertebrae with bone cutting forceps and expose the spinal cord.
- Remove the spinal cord with dissection scissors (Figure 2A-c) and forceps (Figure 2A-d).
- Identify the lumbar DRG by counting vertebrae from the last rib (Thoracic Vertebra 13). See Figure 1C for a diagram of the vertebrae positions.
- Collect the bilateral lumbar DRG (L1-L6) with micro-scissors (Figure 2A-f) into a 35-mm culture dish with 2 mL ice-cold serum-free medium. Remove the neuronal fibers (as indicated in Figure 1C) from connecting DRG, then transfer it into the culture dish to improve the purity of the cultures.
NOTE: The collected DRG can be kept in medium on ice for about 1 h. Meanwhile, multiple rats can be euthanized to create a larger pool of DRG.
2. Primary Culture of Rat Lumber DRG
NOTE: The following steps should be performed in a laminar flow hood.
- Prepare culture medium containing 10% fetal bovine serum, 100 mM sodium pyruvate, and 1x penicillin/streptomycin in 1x DMEM-F12.
- Coat the cell-culture treated 24-well plate with 200 µg/mL poly-L-lysine for 2 h then wash with sterilized water.
- Pre-incubate the culture dish with 1 mL culture medium in a 37 °C CO2 incubator before use for least 30 min.
- Transfer the DRG-containing 35 mm dish into a laminar hood and wash the DRG with serum-free medium 3 times by pipette.
NOTE: The outside of the dish should be cleaned with 75% ethanol before transferring into the hood. The 35 mm dish can contain DRG from a number of rats (this will depend on the demands of the experimental design). - Move the DRG (from a single rat or combined from multiple rats) to a new 35 mm culture dish, which contains 2 mL of collagenase type IA (1 mg/mL in serum-free medium) with sterile tweezers (Figure 2A-e).
NOTE: The collagenase solution should be sterilized by passing it through a 0.22 µm syringe filter. - Digest the DRG in the collagenase solution in a 37 °C CO2 incubator for 30 min.
- Remove the collagenase solution and wash the DRG 3 times in 2 mL Hank's balanced salt solution (HBSS).
NOTE: There may be residual fibers or tissues that come off the DRG into the solution. Remove them by pipette with the washing solution. - Add 2 mL pre-warmed 0.05% trypsin-EDTA into the DRG-containing 35 mm dish and digest the DRG in a 37 °C CO2 incubator for 30 min.
- Transfer the 2 mL of DRG-containing solution to a 15 mL centrifuge tube by glass pipette.
NOTE: The DRG might stick to the glass pipette so this step should be performed with care. DRG loss can be avoided by keeping the DRG-containing solution in the tapered end of a glass pipette (about 0.5 mL) and transferring the solution into the centrifuge tube slowly but without pause. - Centrifuge the solution at 290 x g for 5 min at 4 °C. Remove the supernatant and add another 2-mL serum-free medium to resuspend the DRG.
- Repeat step 2.10 2 times but change the serum-free medium to pre-warmed culture medium on the last time.
- Manually triturate the DRG approximately 60 times using a flame-polished Pasteur pipette (length 230 mm and tip head inner diameter 1 mm). See Figure 2B for a photograph comparing the orifice of a flame-polished Pasteur pipette to a non-polished pipette.
NOTE: The inside diameter of the flame-polished Pasteur pipette is approximately 10% smaller than the control pipette and the inside of the tapered end should be smoother. Be careful not to create bubbles when triturating the cells. - Remove the poly-L-lysine-coated dish from the CO2 incubator. Aspirate the incubated culture medium from the dish and seed the dissociated cells onto the coated dish.
- Seed the DRG cells from one rat (bilateral collection from L1-L6, for 12 total DRG) into four wells of a 24-well plate; there are approximately 5 x 104 cells in one well of a 24-well plate.
NOTE: This density is suitable for immunostaining. For Western blot or RNA extraction, seed the DRG cells from one rat (bilateral L1-L6) into one well of a 6-well plate. - Replace the culture medium on the following day with the addition of 10 µM cytarabine (Ara-C) and 100 ng/mL NGF and refresh the medium every two days thereafter.
NOTE: The thoracic DRG also can also be cultured by this protocol, if they have been collected from the thoracic spine.
Representative Results
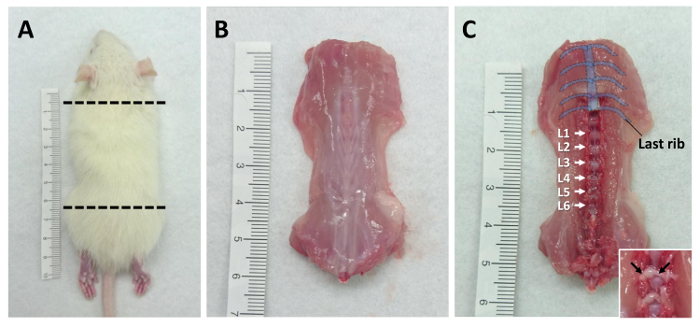
Figure 1: Tissue processing diagrams. Lumbar DRG are collected from 3 week-old rats. (A) The positions where the guillotine should be used to cut the animal are indicated by dotted lines. (B) The dorsal trunk with skin removed and (C) the locations of lumbar DRG (from L1-L6) are shown. The insert represents the DRG and the connecting fibers (which are indicated by the arrows).
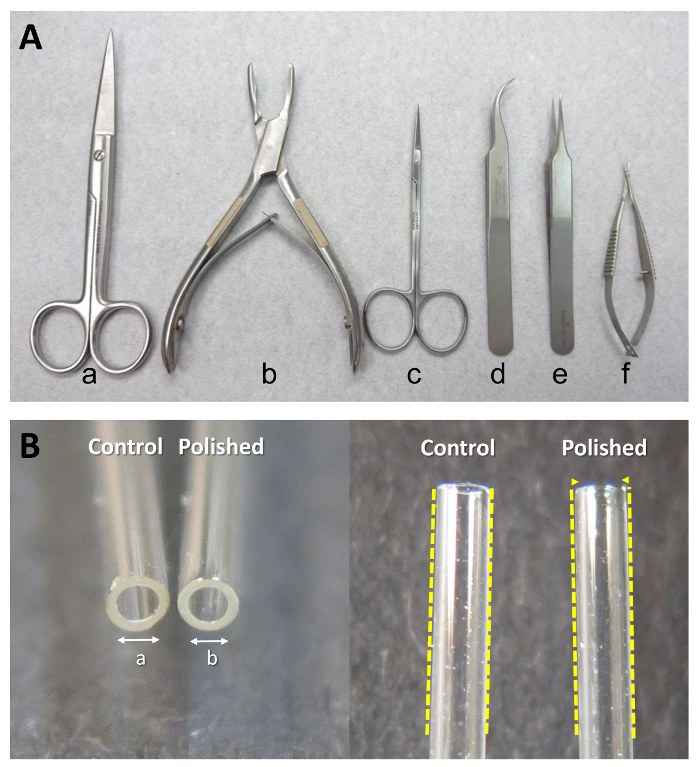
Figure 2: Special equipment needed for isolating DRG primary cultures. (A) Surgical instruments used in the collection of DRG. From left to right: (a) dissection scissors (large), (b) bone cutting forceps, (c) dissection scissors (small), (d, e) point tweezers, and (f) micro-scissors. (B) A regular Pasteur pipette and a flame-polished Pasteur pipette. "a" denotes the inside diameter of regular Pasteur pipette, and "b" denotes the inside diameter of the flame-polished Pasteur pipette. b/a ≒ 0.9.
Declarações
The authors have nothing to disclose.
Materials
| Mixture of tiletamine and zolazepam (Zoletil) | Virbac | Zoletil 50 | anaesthetic |
| Fetal bovine serum | Biological Industries | 04-001-1 | Culture Medium |
| sodium pyruvate | Sigma | S8636 | Culture Medium |
| penicillin/streptomycin | Biological Industries | 03-033-1 | Culture Medium |
| DMEM-F12 | Invitrogen | 12400024 | Culture Medium |
| Poly-I-lysine | Sigma | P9011 | Coating dish |
| Collagenase IA | Sigma | 9001-12-1 | Enzyme digestion |
| Hank's balanced salt solution | Invitrogen | 14170-112 | Culture Medium |
| Trypsin EDTA | Biological Industries | 03-051-5 | Enzyme digestion |
| Pasteur pipette | Hilgenberg | 3150102 | Cell trituration |
| Cytarabine (Ara-C) | Sigma | C6645 | Culture Medium |
| NGF | Millipore | NC011 | Culture Medium |

