Establishing a Müller Glial Cell Culture Obtained from Mouse Retinas
Abstract
Source: Kang, S., et al. Primary Cell Cultures to Study the Regeneration Potential of Murine Müller Glia after MicroRNA Treatment. J. Vis. Exp. (2022).
This video demonstrates the culturing of Müller glial (MG) cells isolated from mouse retinas. Retinas are dissociated into a single-cell suspension using digestive enzymes. Cells are then grown in a medium with a growth factor that promotes MG cell proliferation and neuronal cell mortality. Dead neuronal cells are removed while passaging, and MG cells are grown for further analysis.
Protocol
Disclaimer: All procedures involving sample collection have been performed in accordance with the institute’s IRB guidelines.
1. Retina dissociation
NOTE: All following steps (until cell harvest) need to be carried out in an A2 or B2 biosafety cabinet (BSC).
- Prepare the Papain/DNase I dissociation mixture as follows.
- For six retinas, add 75 µL of DNase I into the tube containing 750 µL of Papain and mix carefully (dissociation mixture).
- For individual sample preparation required for this protocol, split the total volume of 825 µL into three aliquots: 275 µL of the mixture in one 1.5 mL tube per mouse (two retinas). Calculate the required amounts of DNase I and Papain accordingly.
NOTE: Up to six retinas can be dissociated in one tube of Papain/DNase I mixture. However, individual sample preparation results in fewer clumps and better cell growth than combined samples.
- Transfer two retinas to Papain/DNase I dissociation mixture. Use a transfer pipette with an enlarged tip diameter, pick up the retinas, wait until the retinas settle at the bottom of the tip, and then release the retinas without excessive Hanks' Balanced Salt Solution (HBSS) into the tube containing Papain/DNase I mixture.
- Place on a nutator and incubate it for 10 min in a cell culture incubator (37 °C, 5% CO2).
- Dissociate the cells by carefully pipetting up and down (about 20-30 times) with a 1 mL pipette. After cells are dissociated (i.e., resulting in a homogenous solution with no chunks), add 275 µL of Ovomucoid protease inhibitor from the Papain Dissociation Kit to neutralize the Papain. Mix gently by pipetting up and down.NOTE: If six retinas were dissociated in 825 µL of Papain/DNase I mixture, 825 µL of Ovomucoid is required.
- Centrifuge the mixture at 300 x g for 10 min at 4 °C.
- Add epidermal growth factor (EGF, 1 µL per 1 mL of the growth medium, reconstituted at 200 µg/mL in PBS) to the calculated volume of growth medium (1 mL per mouse) pre-warmed at 37 °C.
NOTE: Depending on the experimental design, the proliferation marker 5-ethynyl-2'-deoxyuridine (EdU), as well as 4-Hydroxytamoxifen (4-OHT) or other required factors can be added at the beginning of the culture period. - Remove the tubes carefully from the centrifuge. Do not touch the pellet at the bottom of the tube.
- Remove the supernatant carefully and entirely. Resuspend the cell pellet with 500 µL of EGF-supplemented growth medium.
- Transfer the cell suspension into one well of the labeled 12-well plate (Figure 1C). Rinse the tube with another 500 µL of the EGF-supplemented growth medium and add it to the well (total volume of 1 mL per well).
- Repeat steps 1.8 and 1.9 with all other samples.
- Rock the well plate three times carefully (back and forth; left, and right). Place the plate into the incubator (37 °C, CO2).
NOTE: If transgenic mice are used, perform genotyping for every animal (Figure 1D). Identify Cre recombinase positive and negative mice and label the plate accordingly. For this protocol, only cells of Cre recombinase positive reporter mice were used for the next steps. Cells of Cre negative specimens are frozen and used for other applications.
2. Growth phase
NOTE: The growth phase has a duration of about 4-5 days (Figure 2B). For adding liquids to wells containing cells, the pipette needs to point to the wall of the well and the liquid needs to be released slowly to avoid cell detachment. Do not pipette directly on top of the cells.
- One day after dissociation, remove the medium and add 1 mL of fresh EGF-supplemented growth medium.
- On day 3, remove the medium and add 1 mL of HBSS (room temperature) to remove cell debris. Rock gently back and forth left to right. Remove HBSS, repeat the wash step, and add 1 mL of pre-warmed EGF-supplemented growth medium.
- Monitor the cells every day and evaluate their growth status until the cells reach 90%-100% confluency. Figure 3 shows an example of good MG growth over time. Check for possible contamination or cell death. Discard contaminated cultures.
NOTE: To monitor and record cell state, take images at various magnifications using a light microscope with an attached camera and 4x, 10x, or 20x objectives. In this study, a fluorescence microscope is used.
3. Preparation of coverslips with poly-L-ornithine (Poly-O) and Laminin coat
NOTE: This step is only necessary if immunofluorescent labeling and confocal laser-scanning microscopy are performed. Round glass coverslips (12 mm diameter) are required for proper imaging. The coating protocol can also be found in the neuronal medium datasheet (see Table of Materials).
- Place sterile coverslips carefully in the center of every well of a 24-well plate using sterile Dumont #2AP forceps.
NOTE: Place coverslips in the center of the well. Placing coverslips close to the wall of a well will cause surface tension issues for the following steps. - Thaw a 2.5 mL Poly-O aliquot at room temperature and place 100 µL of it in the center of each coverslip.
- Incubate the well plate for 30 min in a 37 °C incubator.
- Remove Poly-O and wash the well with the coverslip three times with ~1 mL of sterile water.
- Let the well plate dry overnight in the BSC. Thaw 2.5 mL of Laminin at 4 °C overnight.
- The next morning, add 100 µL of Laminin in the center of each coverslip and incubate for 4 h in a 37 °C incubator.
- Remove the Laminin carefully and entirely.
- Keep the plate at 4 °C if passaging cannot be performed immediately.
NOTE: The coated coverslips in prepared plates can be kept for a few days at 4 °C.
4. Cell passage to remove neuronal survivors
NOTE: Cell passage is required to remove neuronal cells, not to increase the cell population. Glia divides only a few times and will not grow further after passage. Do not dilute cell suspensions. The cells of one confluent well of a 12-well plate can be distributed onto one well of a 12-well plate or two wells of a 24-well plate. When coated coverslips are used, only about one-third of the coverslip is coated. Therefore, six coverslips sitting in a 24-well plate, with confluent cells (~80%-90%) can be obtained from one well of confluent cells of a 12-well plate. Other ratios can be chosen to increase or decrease cell density as well. For this protocol, one Cre+ reporter mouse is used [one experiment, two treatments: miR-25 or control-miR; technical replicates n = 3 (three coverslips per treatment), biological replicate n = 1]. The number of technical and biological replicates can be defined differently depending on the experimental design.
- Check whether the cells are 90%-100% confluent (also at the margin of the well; Figure 3E,F).
- Remove the medium and add 1 mL of HBSS (room temperature) to wash. Gently rock the plate (back and forth, left and right). Remove HBSS completely.
- Add 500 µL of a pre-warmed trypsin-containing solution (pre-warmed at 37 °C in a metal bead bath) to detach the cells from the well. Rock gently (back and forth, left to right) and incubate for 2 min in a 37 °C incubator.
- Move the plate from the incubator to BSC. While tilting, aspirate the trypsin-containing solution and disperse it carefully and slowly over the well several times until the cells detach completely. Hold the plate against the light and make sure no cells are left at the bottom.
- Transfer this cell suspension to a sterile 1.5 mL tube. Centrifuge at 300 x g for 8 min at 4 °C.
- Remove the supernatant and carefully resuspend the cell pellet by adding 600 µL (100 µL per well for 6 wells/coverslips) of pre-warmed growth medium (supplemented with EGF; 1:1000). and pipetting up and down ~30-40 times.
NOTE: The cells can be frozen at this time at -80 °C or in liquid nitrogen and defrosted following standard protocols for thawing cell lines. If cells are frozen, they will not be resuspended in an EGF-supplemented medium (growth medium). They will be resuspended in a basic medium (without EGF) and freezing solution (1/1 ratio). - Seed cells by placing 100 µL of the 600 µL cell/media suspension in the center of six coated coverslips (see step 5) of the 24-well plate. Place the plate carefully in the incubator and let the cells settle.
NOTE: 100 µL of the 600 µL cell suspension (harvested from one well of a 12-well plate) will result in 90%-100% cell confluency, which is required for transfection. - Check the cells after 3 h to see whether they have settled on the coverslip. Add 400 µL of growth medium supplemented with EGF.
NOTE: Cells are usually ready for transfection the following day (Figure 3G). If not confluent (90%-100%), leave them for another day. If still not confluent, do not use them for transfection. If other downstream applications are conducted, such as miRNA profiling, RNA-Seq, RT-qPCR, or western blot, cells need to be passaged into 12-well plates (1:1 ratio; no plate treatment required) and harvested for RNA/protein extraction.
Representative Results
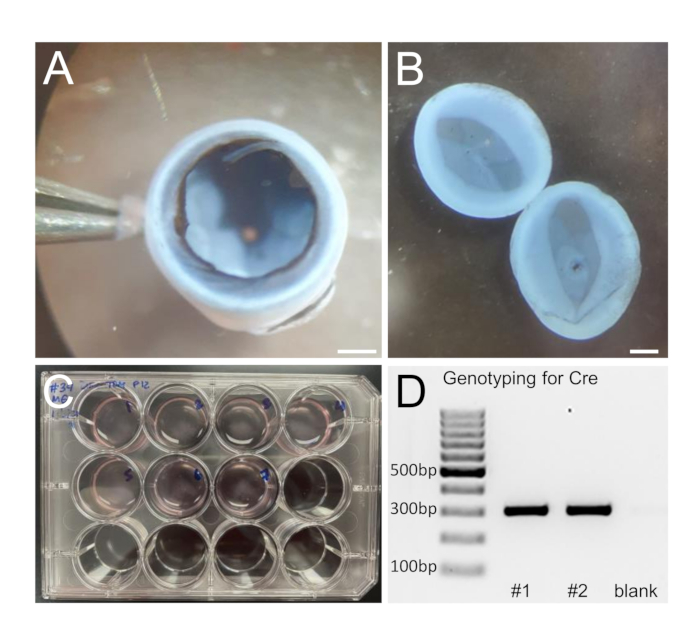
Figure 1: Retinal dissociation and genotyping. (A) Eye cup with removed cornea, lens, iris, and vitreous. (B) Isolated retinas; retinal pigment epithelial (RPE) cells are removed after a thorough wash. (C) 12-well plate with dissociated retinas (two retinas per well). (D) Cutout of a genotyping gel image example. Genotyping is required to identify the mice that have Ascl1 driven Cre recombinase expression and will be used for tracking cell conversion. Scale bars: 1 mm.
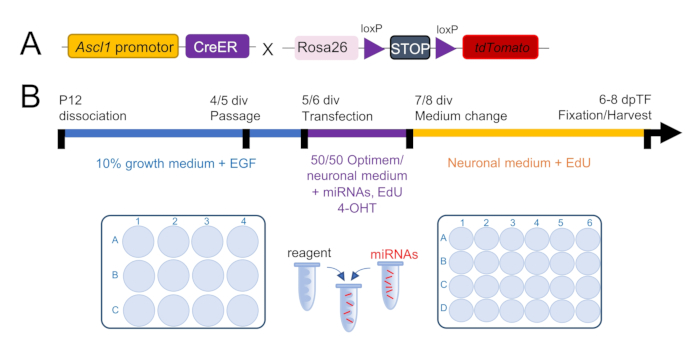
Figure 2: Experimental design and time course of a primary Müller glia culture. (A) Schematic of the Ascl1CreERT:tdTomatoSTOPfl/fl mouse, a retinal progenitor reporter mouse used to track the conversion of Müller glia (MG) into retinal progenitor cells (RPCs). (B) Time course of the culture periods consisting of growth phase (blue, 0-4/5 days in vitro, div), transfection phase (purple, 5/6-7/8 div), and MG conversion phase (yellow, starts 7/8 div). Growth phase: dissociated retinas (two retinas of one mouse) are grown in one well of a 12-well plate in a growth medium. Around day 4/6, cells are passaged into a 24-well plate that contains coated coverslips. Transfection phase: 5-7 div (1 day after passage) cells are transfected with miRNAs for 2 days in transfection medium. Cre recombinase is activated with 4-Hydroxytamoxifen (4-OHT). Cell proliferation is tracked with EdU. MG conversion phase starts 1 day after transfection. Cells are now grown in the neuronal medium until harvest (6-7 days post transfections, dpTF).
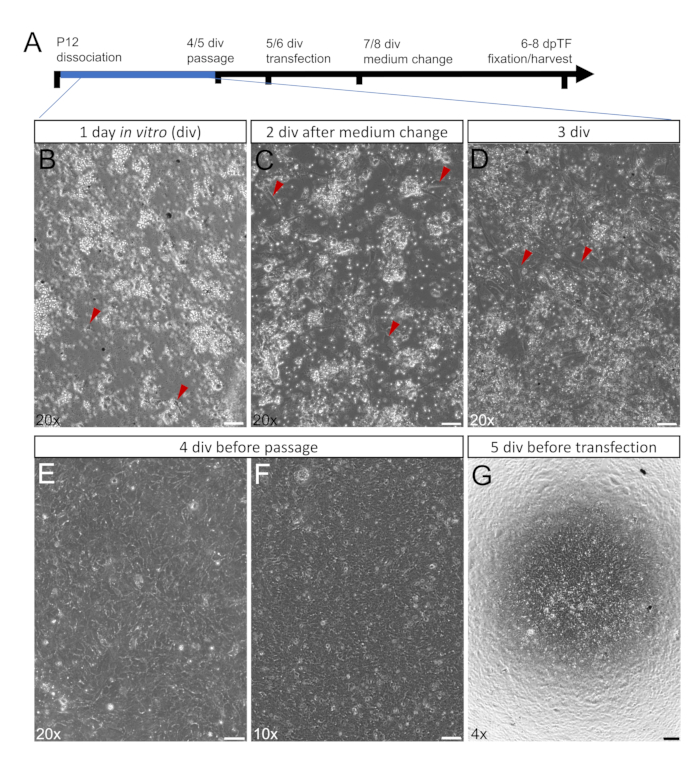
Figure 3: Primary Müller glia during the growth phase. (A) Time course of culture periods during the growth phase (0-4/5 days in vitro (div)) is highlighted in blue. (B-G) Live images (phase) of Müller glia (MG) during the growth phase after 1 div (B), 2 div after the medium change (C), 3 div (D), 4 div before passage (E,F) and 5 div, 1 day after passage and before transfection (G). MG cell bodies are indicated by red arrowheads. After 3 div, cultures are 60%-80% confluent (D), after 4-5 div, cultures are 90%-100% confluent and ready to be passaged (E-F). After passage on coverslips, cell cultures need to be 80%-90% confluent for subsequent transfection (G). Scale bars: 50 µm (A-D), 100 µm (E), 200 µm (F).
Declarações
The authors have nothing to disclose.
Materials
| Animals | |||
| Ascl1-CreERT mouse Ascl1tm1.1(Cre/ERT2)Jejo/J | Jax laboratories | #012882 | Ascl1-CreERT mice were crossed with tdTomato mice |
| tdTomato-STOPfl/fl mouse B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | Jax laboratories | #007914 | Genotyping is requried to identify Ascl1CreER positive mice |
| Reagents | |||
| Poly-L-ornithine hydrobromide | Sigma-Aldrich | P4538-50MG | reconstituted in steriled water, frozen aliquots |
| N-2 Supplement | Fisher Scientific | 17-502-048 | frozen aliquots |
| Neurobasal Medium | Fisher Scientific | 21-103-049 | used for growth medium in section 1.1, store at 4 °C |
| Papain Dissociation System | Worthington Biochemical | LK003153 | reconstituted in Earle's Balanced Salt Solution, frozen aliquots |
| Penicillin Streptomycin | Fisher Scientific | 15-140-122 | frozen aliquots |
| L-Glutamine | Fisher Scientific | 25-030-081 | frozen aliquots |
| HBSS | Fisher Scientific | 14-025-134 | store at 4 °C |
| Fetal Bovine Serum (FBS) | Fisher Scientific | MT35010CV | frozen aliquots |
| plasticware/supplies | |||
| 0.6 mL microcentrifuge tube | Fisher Scientific | 50-408-120 | |
| 1.5 mL microcentrifuge tube | Fisher Scientific | 50-408-129 | |
| 10 µL TIP sterile filter Pipette Tips | Fisher Scientific | 02-707-439 | |
| 100 µL TIP sterile filter Pipette Tips | Fisher Scientific | 02-707-431 | |
| 1000 µL TIP sterile filter Pipette Tips | Fisher Scientific | 02-707-404 | |
| 2.0 mL microcentrifuge tube | Fisher Scientific | 50-408-138 | |
| 20 µL TIP sterile filter Pipette Tips | Fisher Scientific | 02-707-432 | |
| Adjustable-Volume Pipettes (2.5, 10, 20, 100, 200, & 1000 µL) | Eppendorf | 2231300008 | |
| Disposable Transfer Pipets | Fisher Scientific | 13-669-12 | |
| Multiwell Flat-Bottom Plates with Lids, No. of Wells=12 | Fisher Scientific | 08-772-29 | |
| Multiwell Flat-Bottom Plates with Lids, No. of Wells=24 | Fisher Scientific | 08-772-1 | |
| PIPET sterile filter 10ML Disposable Serological Pipets | Fisher Scientific | 13-676-10J | |
| PIPET sterile filter 50ML Disposable Serological Pipets | Fisher Scientific | 13-676-10Q | |
| PIPET sterile filter 5ML Disposable Serological Pipets | Fisher Scientific | 13-676-10H | |
| Powder-Free Nitrile Exam Gloves | Fisher Scientific | 19-130-1597B | |
| Round coverslips (12 mm diameter, 0.17 – 0.25 mm thickness) | Fisher Scientific | 22293232 | |
| Vacuum Filter, Pore Size=0.22 µm | Fisher Scientific | 09-761-106 | |
| equipment | |||
| 1300 B2 Biosafety cabinet | Thermo Scientific | 1310 | |
| All-in-one Fluorescence Microscope Keyence BZ-X 810 | Keyence | 9011800000 | |
| Binocular Zoom Stereo Microscope | Vision Scientific | VS-1EZ-IFR07 | |
| Disposable Petri Dishes (100 mm diameter) | VWR | 25384-088 | |
| Dumont #5 Forceps – Biologie/Titanium | Fine Science Tools | 11252-40 | |
| Dumont #55 Forceps – Biologie/Inox | Fine Science Tools | 11255-20 | |
| Dumont #7 curved Forceps – Biologie/Titanium | Fine Science Tools | 11272-40 | |
| Eppendorf Centrifuge 5430 R | Eppendorf | 2231000508 | |
| Water Jacketed CO2 Incubator | VWR | 10810-744 |

