Obtaining Horizontal Mouse Hippocampal Brain Slices
Abstract
Source: Van Hoeymissen, E., et al. Horizontal Hippocampal Slices of the Mouse Brain. J. Vis. Exp. (2020)
This video demonstrates a detailed protocol for preparing horizontal hippocampal brain slices from mice, focusing on preserving the integrity of hippocampal fiber pathways. The process involves carefully bisecting and securing the brain, using a vibratome for slicing, and maintaining optimal tissue conditions with a carbogenated fluid, resulting in hippocampal brain slices ready for further experimentation.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Preparation of high-sucrose slice solution and artificial cerebrospinal fluid (ACSF)
- Prior to experimental day
- Prepare 1 L of 10x slice pre-solution with laboratory grade Type 1 water containing (in mM): 25 KCl, 20 CaCl2, 10 MgSO4, 12.5 KH2PO4 (Table 1). In order to prevent calcium phosphate precipitation, slowly mix the chemicals in a beaker pre-filled with 800 mL H2O while constantly stirring with a magnetic stirrer. Store the solution at 4 °C or room temperature (RT).
- On the experimental day
- Prepare 1 L of 1x ACSF with laboratory grade Type 1 water containing (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 25 Glucose (Table 2). Use a vapor pressure osmometer to validate the osmolarity between 305–315 mOsm (pH ~ 7.55–7.6).
NOTE: In order to prevent calcium phosphate precipitation, slowly mix all solid chemicals in a beaker pre-filled with 800 mL of H2O while constantly stirring with a magnetic stirrer. Add MgSO4 and CaCl2 at the very end, slowly dripping in the necessary amount from 1 M stock solutions. - Continuously bubble 1x ACSF solution at RT with carbogen to set pH between 7.3–7.4.
NOTE: If the pH is slightly too high or too low, small adjustments in the carbogenation strength would be sufficient. If the pH is higher than 7.45 with carbogenation, adjust it by adding few drops of 1 M NaH2PO4 solution. - Prepare 250 mL (per brain) of 1x high-sucrose slice solution in a beaker containing 25 mL of 10x slice pre-solution and (in mM): 252 Sucrose, 26 NaHCO3, and 10 Glucose (Table 3). Verify that the osmolarity is between 320–325 mOsm (pH ~ 7.55–7.6).
- Bubble the high-sucrose slice solution for 10–15 min with carbogen to control the pH between 7.3–7.4.
NOTE: If the pH is higher than 7.45 with carbogenation, adjust it by adding a few drops of 1 M KH2PO4 solution. - Store the high-sucrose slice solution for 20–30 min in the ultra freezer (-80 °C) until it is partially frozen.
- Prepare 1 L of 1x ACSF with laboratory grade Type 1 water containing (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 25 Glucose (Table 2). Use a vapor pressure osmometer to validate the osmolarity between 305–315 mOsm (pH ~ 7.55–7.6).
2. Dissection and positioning of the murine brain
- Transfer the brain to the chilled 35 mm culture dish and remove all hair or blood particles from the tissue by gently washing the brain with an ACSF-filled Pasteur pipette (blood has cytotoxic effects on brain tissue).
- Transfer the brain with the help of the spatula on the soaked filter paper on top of the chilled 90 mm culture dish (Figure 1A).
- Use a blade to cut the brain in half, separating the two hemispheres, and place both hemispheres on the freshly cut medial side.
- Use a blade to remove the dorsal part (5%–10%) of the brain from each hemisphere with a parallel cut to the dorsal top (Figure 1C)and place both hemispheres on the freshly cut side with the ventral part of the brain facing upwards.
- Position a drop of super glue on the specimen plate and spread out properly with a pipette tip to accommodate both hemispheres.
- Use a filter paper strap to pick up one hemisphere with capillary forces by touching the ventral side with the filter paper strap, thereby not damaging the tissue.
- Use another filter paper strap to carefully semi-dry the dorsal side of the brain before positioning the hemisphere, dorsal side down, on top of the glue on the specimen plate. Repeat the same procedure with the second hemisphere.
NOTE: The hemispheres should be positioned in horizontal alignment in a mirrored fashion on the vibratome plate, with the rostral sides pointing toward the outside and the caudal sides facing (but not touching) each other in the middle. The medial sides of both hemispheres should point toward the vibratome blade and the lateral sides toward the experimenter (Figure 1D). - Place the specimen plate in the slicing chamber and quickly, but carefully, cover it with ice-cold high-sucrose slice solution slush. As soon as the solution touches the glue, it will solidify and properly glue the hemispheres to the specimen plate.
- Assure that the hemispheres are properly covered with high-sucrose slice solution and confirm that the solution is bubbled with carbogen.
NOTE: The entire dissection procedure should be performed as fast as possible. Please ensure that the brain does not stay without the supply of oxygen for a very long time. It should take only around 1–1.5 min from decapitation to brain submersion in the high-sucrose slice solution slush. This is the most critical requirement for acute brain slice preparations in order to warrant high quality of the slice.
3. Horizontal slicing of the brain
- Position the vibratome blade in front of the medial side of the hemispheres and lower it to the same height as the ventral sides of the hemispheres that are now facing upwards. Lower the blade with the help of the vibratome control to 600 µm further in the dorsal direction and start slicing. The blade should hit the tissue (if not, reverse the blade and lower it a bit more). Slice until the first two slices are completely separated from the two hemispheres.
- Reverse the blade and lower another 300 µm and slice again.
- When the hippocampus becomes visible (use the mouse brain atlas for help, if necessary) (Figure 1E) collect the slices with the widened plastic Pasteur pipette. Collect the slices until the caudate putamen becomes visible next to the hippocampus. Typically, between 8–12 slices of 300 µm (4–6 per hemisphere) can be collected for the mouse brain.
- Use the plastic Pasteur pipette to collect the slices and transfer them to the recovery chamber in the water bath (Figure 1F) (collect slices after each round of slicing to prevent them from floating around in the slice chamber).
NOTE: Work as fast as possible and ensure that the high-sucrose slice solution is ice-cold and carbogenated during the entire procedure. If necessary, refill the ice surrounding the slice chamber.
4. Recovery of brain slices for electrophysiological recordings
- Leave the slices in the ACSF-filled recovery chamber in the 32 °C water bath for 1 h.
NOTE: Recovery chambers are also commercially available. - Take the recovery chamber out of the water bath.
- Place the slices at RT for at least another 30 min for recovery before starting any further application.
Buffer and Media Recipes.
Table 1: 10 x slice pre-solution (1 L).
| Compound | Concentration (mM) | Molecular weight (g/mol) | Amount (g) |
| KCl | 25 | 74.55 | 1.86 |
| CaCl2 * 2H2O | 20 | 147.01 | 2.94 |
| MgSO4 * 7H2O | 10 | 246.48 | 2.46 |
| KH2PO4 | 12.5 | 136.08 | 1.7 |
Table 2: 1x ACSF (1 L) (osmolarity between 305–315 mOsm).
| Compound | Concentration (mM) | Molecular weight (g/mol) | Amount (g) |
| NaCl | 125 | 58.44 | 7.3 |
| KCl | 2.5 | 74.55 | 0.19 |
| CaCl2 * 2H2O | 2 | from 1 M CaCl2 solution | 2 mL |
| MgSO4 * 7H2O | 1 | from 1 M MgSO4 solution | 1 mL |
| NaH2PO4 * 2H2O | 1.25 | 156.02 | 0.2 |
| NaHCO3 | 26 | 84.01 | 2.18 |
| Glucose * H2O | 25 | 198.17 | 4.95 |
Table 3: 1x high-sucrose slice solution (250 mL) (osmolarity between 320–325 mOsm).
| Compound | Concentration (mM) | Molecular weight (g/mol) | Amount (g) |
| 10x slice presolution | N/A | N/A | 25 mL |
| Sucrose | 252 | 342.3 | 21.57 |
| NaHCO3 | 26 | 84.01 | 0.55 |
| Glucose * H2O | 10 | 198.17 | 0.49 |
Representative Results
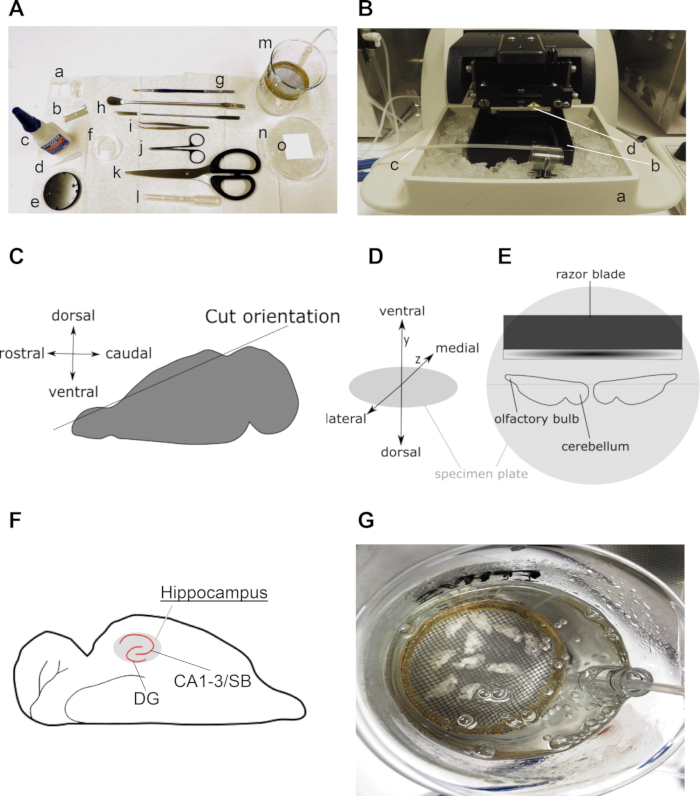
Figure 1: Detailed information on the preparation of horizontal hippocampal brain slices. (A) Image of tools required for dissection and slicing of the rodent brain: (a) ±2 cm long and ±0.5 cm wide straps of filter paper (e.g., grade 413); (b) blade; (c) super glue; (d) pipette tip; (e) specimen plate (comes with vibratome); (f) 35 mm culture dish; (g) fine brush; (h) spatula; (i) curved forceps; (j) dissection scissors; (k) strong scissors (blade length above 10 cm); (l) plastic Pasteur pipette with wide opening (between 0.6 to 0.8 cm in diameter); (m) recovery chamber (self-made with 250 mL beaker, plastic ring, nylon mesh, piece of a 10 mL serological pipette); (n) 90 mm culture dish filled with ice and (o) square of filter paper on top of the chilled culture dish. (B) Picture of a vibratome with (a) holder of slice chamber filled with ice; (b) slice chamber; (c) carbogen line and (d) slicing razor blade. (C) Cartoon illustrating the orientation of the cut of the dorsal side of one hemisphere in order to prepare the brain for horizontal slicing (see step 2.4). (D) Isometric projection of the brain orientation on the specimen plate of the vibratome. (E) Cartoon illustrating a top view of the position of the two hemispheres on the specimen plate. (F) Cartoon showing the position of the hippocampus in a horizontal brain slice. The dentate gyrus (DG) and Cornu Ammonis (CA)–Subiculum (SB) regions of the hippocampus are indicated. (G) Picture of a recovery chamber with carbogenated ACSF containing ten freshly sliced horizontal brain slices.
Declarações
The authors have nothing to disclose.
Materials
| Carbogen tank | Air Liquide | Alphagaz mix B50 | Gasmixture CO2/O2: 5/95, purity 5 |
| Culture dish (35 mm) | Corning Life Sciences | 353001 | https://ecatalog.corning.com/life-sciences/b2c/US/en/Cell-Culture/Cell-Culture-Vessels/Dishes%2C-Culture/Falcon®-Cell-Culture-Dishes/p/353001 |
| Culture dish (90 mm) | Thermo Fisher Scientific | 101VR20 | https://www.thermofisher.com/order/catalog/product/101R20#/101R20 |
| Curved forceps | Fine Science tools | 11270-20 | https://www.finescience.de/de-DE/Products/Forceps-Hemostats/Dumont-Forceps/Dumont-7b-Forceps/11270-20 |
| D-(+)-Glucose monohydrate | Sigma Aldrich | 16301 | https://www.sigmaaldrich.com/catalog/product/sial/16301?lang=en®ion=BE |
| Filter paper | VWR | 516-0818 | grade 413 |
| Fine brush | Raphael Kaerell | 8204 | Size #1 |
| Loctite 406 | Henkel Adhesive technologies | Loctite 406 | Super glue |
| Potassium chlorid | Chem-lab | CL00.1133 | https://www.chem-lab.be/#/en-gb/prod/1393528 |
| Potassium dihydrogen phosphate | Merck | 104873 | https://www.merckmillipore.com/BE/en/product/Potassium-dihydrogen-phosphate,MDA_CHEM-104873?ReferrerURL=https%3A%2F%2Fwww.google.com%2F |
| Razor blade to prepare hemispheres | SPI supplies | Safety Cartridge Dispenser – Pkg/10 | GEM Scientific Single Edge Razor Blades |
| Razor blade for vibratome | Ted Pella Inc | 121-6 | double edge breakable style razor blades (PTFE-coated stainless steel) |
| Recovery chamber | home made – Generic | N/A | to collect and store brain slices in (see details in manuscript) |
| Scissors | Any company | N/A | Blade should be well sharpened and at least 15 cm long for easy decapitation |
| Sodium dihydrogen phosphate dihydrate | Merck | 106342 | https://www.merckmillipore.com/BE/en/product/Sodium-dihydrogen-phosphate-dihydrate,MDA_CHEM-106342?ReferrerURL=https%3A%2F%2Fwww.google.com%2F |
| Sodium hydrogen carbonate | Alfa Aesar | 14707 | https://www.alfa.com/en/catalog/014707/ |
| Sodium chlorid | Fisher Scientific | S/3160/60 | https://www.fishersci.co.uk/shop/products/sodium-chloride-certified-ar-analysis-meets-analytical-specification-ph-eur/10428420 |
| Spatula | Sigma Aldrich | S9147-12EA | https://www.sigmaaldrich.com/catalog/product/sigma/s9147?lang=en®ion=BE |
| Sucrose | VWR International Ltd. | 102745C | https://es.vwr-cmd.com/ex/downloads/magazine/lupc_userguide_uk.pdf |
| Tubing for carbogen, perfusion and suction lines 1 | Warner Instruments | 64-0167 | Tygon tubing (TY-50) for standard valve systems |
| Tubing for carbogen, perfusion and suction lines 2 | Fisher Scientific | 800/100/200 & 800/100/280 | Smiths Medical Portex Fine Bore LDPE Tubing |
| Vibratome | Leica | 14912000001 | Semi-automatic vibrating blade microomei VT1200 |
| Water bath | Memmert | WNB 7 | https://www.memmert.be/wp-content/uploads/2019/09/Memmert-Waterbath-WNB-7.en_.pdf |
| Water purification system | Merck | Synergy millipore | to obtain highly purified water |

