Human Pluripotent Stem Cell Differentiation in Neurons Using Engineered Lentiviruses
Abstract
Source: Assetta, B., et al., Generation of Human Neurons and Oligodendrocytes from Pluripotent Stem Cells for Modeling Neuron-Oligodendrocyte Interactions. J. Vis. Exp. (2020).
This video demonstrates the process of inducing human pluripotent stem cells to differentiate into neurons by transfecting them with engineered lentiviruses carrying a neurogenic transcription factor gene under the control of tetracycline-responsive element, followed by treatment with a tetracycline derivative and maturation medium.
Protocol
1. Human neuron induction from human pluripotent stem cells
- Lentivirus preparation (~5 days, detailed protocol as described previously)
- Plate ~1 million HEK293T cells in each T75 flask to have them ~40% confluent when performing transfection. Transfect them with plasmids expressing tetracycline-inducible Ngn2 and puromycin-resistant gene (PuroR; under the same TetO promoter control), rtTA, and the three helper plasmids pRSV-REV, pMDLg/pRRE, and VSV-G (12 µg of lentiviral vector DNA and 6 µg of each of the helper plasmid DNA). Prepare at least three flasks per lentivirus preparation. Use PEI for transfection following the manufacturer’s instructions. Change the media after 16 h and discard.
- Harvest released viral particles by collecting culture media every day and replace with fresh media for 3 days. Pool the collected media containing viral particles for purification. Filter the virus through a 0.22 µm filter and centrifuge at 49,000 x g for 90 min. Resuspend the pellet in the appropriate volume of PBS-glucose (~150 µL).
- Neuron Induction (~5 days)
NOTE: This induction protocol (Figure 1A; flow diagram) is highly effective for both iPS and ES cells of validated pluripotency (which can be assayed by immunohistochemistry staining of well-characterized pluripotency markers; Figure 1B).- Use commercially available H1 human ES cells at the passage of 52 (see Table of Materials). Culture the cells on extracellular matrix solution-coated 6-well plates (~0.5 mg of matrix solution per 6-well plate; see Table of Materials) using ES cell maintenance medium (see Table of Materials) and incubate the plates at 37 °C with 5% CO2.
- On Day -2, detach ES cells (80% confluent) with 1 mL of cell detachment solution (see Table of Materials) and incubate at room temperature for 10 min. Transfer the cells to a tube; wash the well with 2 mL of media and combine in the same tube. Centrifuge at 300 x g for 5 min, resuspend the pellet in media, and plate the cells onto matrix-coated 6-well plates at the seeding density of 1 x 105 cells per well.
- On Day -1, add lentiviruses expressing Ngn2 plus PuroR and rtTA together with polybrene (8 µg/ml) to the ES cells in fresh ES cell maintenance medium (see Table of Materials). The exact amount of viruses should be determined by actual titers or the titration. We typically add 5 µL each virus per well in a 6-well plate.
- On Day 0, add Doxycycline (2 µg/mL to activate Ngn2 expression) in DMEM-F12 medium with N2 supplement without morphogens.
- On Day 1, add Puromycin in fresh medium of DMEM-F12 plus N2 and doxycycline, to the final concentration of 1 µg/mL medium. Select the transduced cells in Puromycin for at least 24 h. Higher Puromycin concentration (up to 5 µg/mL) and longer selection period (up to 48 h) may be required to adequately remove the under-transduced cells if the virus titer is low.
- On Day 2, detach differentiating neurons with cell detachment solution (see Table of Materials), re-plate them on 24-well plates (between 80,000–200,000 cells/well) coated with matrix solution (see Table of Materials), and maintain them in NBA/B27 medium without doxycycline. The seeding density is critical.
- At this stage, detached neurons can be frozen in a specialized commercial freezing medium (see Table of Materials) and stored in liquid nitrogen for up to 3 months. Pure neurons can be plated accounting for the typical ~15%–20% cell death post-thaw, cultured alone or co-cultured with other brain cell types.
- Culture pure iNs on the plates coated with extracellular matrix-based solutions as instructed by the manufacturer (see Table of Materials). The characteristic pyramidal morphology should be apparent by Day 4 (and Day 6; Figure 1C). The synapse formation can be detected as early as Day 14 to 16 and is prominent at Day 24 by immunohistochemical staining with standard pre- and post-synaptic markers. (Figure 1D; labeled with the pre-synaptic marker Synapsin 1 and the dendritic marker Map2).
Representative Results
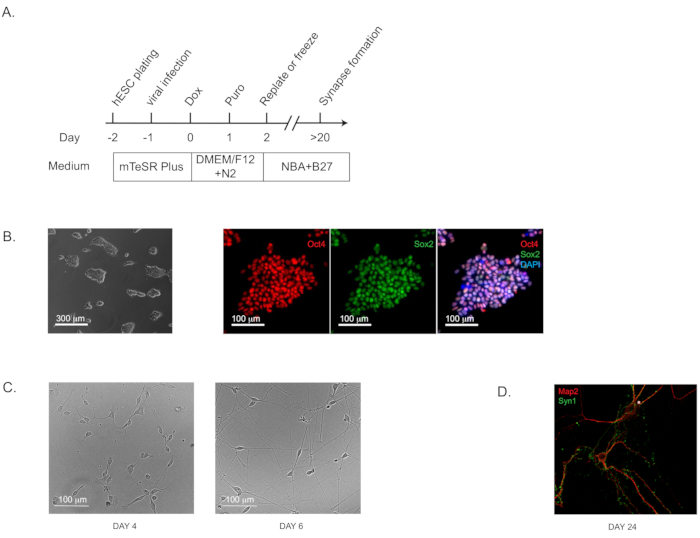
Figure 1: Direct Generation of human-induced neurons (iNs) from hPSCs. (A) Flow diagram of iN generation. (B) Representative bright field and immunofluorescence images of the starting culture of human pluripotent stem cells (H1) to confirm the pluripotency. Oct4 is shown in red and Sox2 in green. (C) Representative bright field images of iNs at Day 4 and Day 6. (D) The characteristic morphology for dendritic arborization and synapse puncta in iNs grown in pure culture for 24 days and stained by immunofluorescence staining for dendritic marker Map2 and pre-synaptic marker Synapsin 1 (Syn1).
Declarações
The authors have nothing to disclose.
Materials
| Accutase | STEMCELL Technologies | 7920 | |
| B27 supplement | ThermoFisher | 17504044 | |
| DMEM/F12 medium | STEMCELL Technologies | 36254 | |
| DMSO | ThermoFisher | D12345 | |
| Doxycycline | MilliporeSigma | D3072 | |
| H1 human ES cells | WiCell | WA01 | |
| Matrigel | Corning | 354234 | |
| mTeSR plus | STEMCELL Technologies | 5825 | |
| N2 supplement | ThermoFisher | 17502001 | |
| Neurobasal A medium | ThermoFisher | 10888-022 | |
| Non Essential Amino Acids | ThermoFisher | 11140-050 | |
| PEI | VWR | 71002-812 | |
| pMDLg/pRRE | Addgene | 12251 | |
| Polybrene | MilliporeSigma | TR-1003-G | |
| pRSV-REV | Addgene | 12253 | |
| Puromycin | ThermoFisher | A1113803 | |
| STEMdiff Neural Progenitor Freezing Media | STEMCELL Technologies | 5838 | |
| STEMdiff SMADi Neural Induction Kit | STEMCELL Technologies | 8581 | |
| Tempo-iOlogo: Human iPSC-derived OPCs | Tempo BioScience | SKU102 | |
| TetO-Ng2-Puro | Addgene | 52047 | |
| VSV-G | Addgene | 12259 |

