Co-culturing of a Dorsal Root Ganglion Explant with Schwann Cells for Neuron Myelination
Abstract
Source: Blusch, A. et al., In Vitro Myelination of Peripheral Axons in a Coculture of Rat Dorsal Root Ganglion Explants and Schwann Cells. J. Vis. Exp. (2023)
This video demonstrates the coculturing model of a dorsal root ganglion explant and Schwann cells. In coculture conditions, ascorbic acid promotes the differentiation of Schwann cells into myelinating forms. Myelinating Schwann cells wrap the axons multiple times to create a myelin sheath, which is essential for nerve function.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Schwann cell culture
- Coating for Schwann cell culture
- Coat the cell culture dishes under sterile conditions. Apply 2 mL of 0.01% poly-L-lysine (PLL) to two 60 mm tissue culture (TC) dishes each and incubate overnight at 4 °C.
- Remove the PLL, wash the TC dishes 2x with distilled water, and incubate with 2 mL of 1 µg/cm2 laminin overnight at 4 °C. Wash the TC dishes 2x with aqua dest, and let the plates air-dry.
- Medium preparation for Schwann cell culture
- Prepare 50 mL of Schwann cell medium by adding 10% heat-inactivated fetal calf serum (FCS), 2 µM forskolin, 10 nM neuregulin, and 50 µg/mL gentamycin to Dulbecco′s Modified Eagle′s Medium (DMEM)/F-12 (high glucose) under sterile conditions.
- Prepare 70 mL of Leibovitz's L-15 medium with 50 µg/mL gentamycin under sterile conditions.
- Sciatic nerve preparation
NOTE: All sciatic nerve preparation steps are performed under a clean bench.- Prepare one 100 mm TC dish with 5 mL of ice-cold Dulbecco's phosphate-buffered saline (DPBS) without Ca2+ and Mg2+, one 100 mm TC dish with 5 mL of ice-cold Leibovitz's L-15 medium, and one 100 mm TC dish with 5 mL of ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin.
- Clean all the instruments by autoclaving. Spray the instruments and working area with 70% ethanol.
- Euthanize five 3-week-old male Sprague Dawley rats using CO2 inhalation and decapitation. Spray the rat's torso with 70% ethanol.
- Open the dorsal lower left limb with scissors and remove the biceps femoris muscle carefully. Loosen the sciatic nerve by smooth elevation with curved forceps, ensuring not to bruise the nerve.
- Hold the most proximal part of the nerve with the curved forceps to straighten the nerve, and clip the nerve as high as possible using scissors. Then, clip the nerve close to the sacral plexus and the paw with scissors. Repeat steps 1.3.4-1.3.5 for the right side.
NOTE: While opening the limb, take care not to bruise any blood vessels. - Using forceps, put the left and right sciatic nerves into a 100 mm TC dish with ice-cold DPBS.
- Sciatic nerve refurbishment
- Use forceps to transfer all the nerves to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin. Continue using a stereomicroscope and remove fat, muscle, and blood vessels from the nerves with two pairs of fine forceps. Grab the nerves with forceps and transfer them to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium.
- Identify the proximal and distal ends of the sciatic nerve. Remove the epineurium with one pair of fine forceps in a proximal to distal direction, while holding the proximal nerve end with the second pair of fine forceps.
- Transfer the purified nerves to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin. Tease the isolated nerve fascicles to separate and isolate single nerve fibers using two pairs of fine forceps.
- Transfer the nerve fibers to a 50 mL tube using a 10 mL serological pipette and take up as little medium as possible. Add 50 mL of Leibovitz's L-15 medium with 50 µg/mL gentamycin to the nerve fibers and slew a few times in the 50 mL tube.
- Enzymatic digestion of the sciatic nerve
NOTE: The next steps (steps 1.5-1.8, 2.1, and 2.2) are performed under sterile conditions.- Prepare the enzymatic digestion solution containing 0.25% dispase II, 0.05% type I collagenase, and 50 µg/mL gentamycin in 10 mL of DMEM (high glucose).
- Centrifuge the tube at 188 x g for 5 min at 4 °C, remove the supernatant with a 25 mL serological pipette ,and transfer the pellet with the remaining Leibovitz's L-15 medium into a 60 mm TC dish using a 1,000 mL pipette.
- Rinse the 50 mL tube with 10 mL of the enzymatic digestion solution and add it to the dish containing the nerve fibers. Distribute the tissue in the dish carefully with the tip of a pipette to maximize the accessible surface for digestion.
- Incubate at 37 °C and 5% CO2 for 18 h, and stop the digestion by adding 10 mL of 40% FCS in Hanks' balanced salt solution, without Ca2+ and Mg2+ (HBSS).
- Cell separation
- Transfer the digested nerves into a 50 mL tube using a serological pipette and centrifuge at 188 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet in 10 mL of DMEM containing 10% FCS and 50 µg/mL gentamycin. Resuspend the pellet 20 times subsequently, using a 10 mL, 5 mL, 2 mL, 1 mL, and 200 µL pipette tip.
- Filter the cell suspension through a 100 µm cell strainer and centrifuge at 188 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet with 4 mL of DMEM containing 10% FCS and 50 µg/mL gentamycin.
- Add 2 mL of the cell suspension to each of the two PLL- and laminin-coated 60 mm TC dishes, and incubate at 37 °C and 5% CO2. Leave the plates untouched for 2 days in the incubator to protect the cells from mechanical stress and to support adherence.
- Schwann cell differentiation
- After 2 days, remove the medium and carefully rinse the plates 2x with DMEM (high glucose), 10% FCS, and 50 µg/mL gentamycin. Afterward, add 2 mL of Schwann cell medium. Replace the Schwann cell medium every 2nd day, and observe the cell appearance and confluency using a microscope.
NOTE: Schwann cells and DRG explants need to be prepared in a timely, coordinated manner for the coculture. Make sure a rat with embryos at E 13.5 is available for DRG preparation when the Schwann cells are close to a confluency of 80%.
- After 2 days, remove the medium and carefully rinse the plates 2x with DMEM (high glucose), 10% FCS, and 50 µg/mL gentamycin. Afterward, add 2 mL of Schwann cell medium. Replace the Schwann cell medium every 2nd day, and observe the cell appearance and confluency using a microscope.
- Cell trypsinization and magnetic separation
NOTE: For exemplary pictures of different Schwann cell culture stages, see Figure 1.- When the cells reach a confluency of about 80% (6-12 days of culture), carefully wash the plates 2x with 3 mL of DPBS and incubate with 2 mL of 0.05% Trypsin/EDTA (ethylenediaminetetraacetic acid) (prewarmed to 37 °C) for 3 min. When the cells detach from the plate bottom, inactivate digestion by the addition of 2 mL of DMEM with 10% FCS and 50 µg/mL gentamycin.
NOTE: Stick to a trypsinization time of strictly 3 min and proceed rapidly afterward. - Resuspend the cell pellet in 2 mL of magnetic cell separation buffer containing DPBS with 0.5% bovine serum albumin (BSA) and 2 nM EDTA. Combine 10 µL of the cell suspension with 10 µL of trypan blue and count the cells using a staining chamber.
- Centrifuge the cell suspension at 188 x g for 10 min at 4 °C and resuspend the cell pellet in 90 µL of magnetic cell separation buffer per 1 x 107 cells. Add 10 µL of Thy-1 microbeads per 1 x 107 cells. Resuspend the solution a few times and incubate for 15 min in the dark at 8 °C.
- Add 2 mL of the magnetic cell separation buffer to the cell suspension and centrifuge at 300 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet in 500 µL of magnetic cell separation buffer.
- Moisten the magnetic cell separation column with 1 mL of the magnetic cell separation buffer. Place the magnetic cell separation column in the magnetic cell separator. Apply the cells to the magnetic cell separation column. Collect the flow through and centrifuge at 300 x g at 4 °C for 10 min.
NOTE: Fibroblasts are positively selected and remain in the column, while Schwann cells pass the column. Fibroblasts can be collected with a stamp (e.g., as a negative control for Schwann cell staining protocols). - Discard the supernatant and resuspend the pellet in 1 mL of the coculture medium (see step 3.1.1). Count the cells after staining with trypan blue and a staining chamber.
- When the cells reach a confluency of about 80% (6-12 days of culture), carefully wash the plates 2x with 3 mL of DPBS and incubate with 2 mL of 0.05% Trypsin/EDTA (ethylenediaminetetraacetic acid) (prewarmed to 37 °C) for 3 min. When the cells detach from the plate bottom, inactivate digestion by the addition of 2 mL of DMEM with 10% FCS and 50 µg/mL gentamycin.
2. DRG explant culture
- DRG growth medium preparation
- Prepare DRG growth medium by adding 2% B27, 2% horse serum, 1% L-glutamine, 0.5% penicillin/streptomycin, and 10 ng/mL nerve growth factor (NGF) to the neurobasal medium. Store the growth medium at 4 °C.
NOTE: The growth medium can be used for 2 days.
- Prepare DRG growth medium by adding 2% B27, 2% horse serum, 1% L-glutamine, 0.5% penicillin/streptomycin, and 10 ng/mL nerve growth factor (NGF) to the neurobasal medium. Store the growth medium at 4 °C.
- Coating for DRG explants
- Incubate the coverslips in 70% ethanol for 1 h and place in the wells of 4-well dishes using curved forceps. After the ethanol has dried, apply 300 µL of 0.2 mg/mL poly-D-lysine (PDL) per well and incubate overnight at 37 °C and 5% CO2.
- Wash the coverslips 3x for 5 min each with DPBS consecutively. Take off the DPBS, apply 300 µL of 1 µg/mL laminin to the coverslips, and incubate overnight at 37 °C and 5% CO2.
- After three washing steps with DPBS for 5 min, replace the DPBS with 190 µL of DRG growth medium. Place the 4-well plates into the incubator at 37 °C and 5% CO2.
- DRG preparation
NOTE: Harvest the DRG of embryonic rats under a clean bench.- Before preparation, clean all the instruments with 70% ethanol. Fill 10 (two per embryo) 35 mm TC dishes with 2 mL of ice-cold HBSS per dish, and two 100 mm TC dishes with 5 mL of ice-cold HBSS per dish.
- Euthanize the pregnant rats (adult female Sprague Dawley rats, E13.5) by CO2 inhalation and decapitation.
- Spray the body with 70% ethanol and open the ventral torso of the rat. Carefully remove the uterus and place it into a 100 mm TC dish with ice-cold HBSS.
- Hold the uterus using curved forceps and open the uterus wall with fine forceps. Remove one amniotic sac and open it carefully by pinching a hole with fine forceps.
- Remove the embryo from the surrounding tissues, cut the umbilical cord, and decapitate the embryo using fine forceps. Place the torso into a 100 mm TC dish filled with HBSS using curved forceps and a spatula.
- Quickly remove all the embryos from the uterus and transfer them into one 100 mm TC dish filled with HBSS. If there are more than five embryos, prepare an additional 35 mm TC dish with 2 mL of HBSS, according to step 2.3.1.
- Gently place one embryo torso into a 35 mm TC dish filled with HBSS using a spatula and curved forceps. Under a stereomicroscope, open the dorsal part of the torso to divide the embryo into two halves using fine forceps and micro scissors. Turn one half to the side and identify the strand of DRG located in a line at the dorsal part of the embryo.
- Cut out the DRG as a whole strand using fine forceps and micro scissors. Place the DRG in a fresh 35 mm TC dish filled with 2 mL of HBSS, and separate a single DRG from the remaining tissue using fine forceps and micro scissors.
- DRG cell culture
- Take 4-well plates containing 190 µL of DRG growth medium from the incubator to the clean bench where the DRG are to be prepared. Transfer a single DRG carefully into one well of a 4-well culture plate using fine forceps and a spatula. Place the DRG into the center of each well, since a central position is important for the attachment of the DRG.
- Work under sterile conditions from now on. Place the explant cultures in the incubator at 37 °C and 5% CO2. The next day, add 50 µL of the DRG growth medium carefully to each well using a 100 mL pipette.
- Observe DRG explant adherence and axon outgrowth using a microscope daily, and discard DRG explants that have detached from the coverslip or failed to outgrow axons on day 3 of culture.
NOTE: DRG explants are very fragile in the first days of culture and need to be handled with care, especially when moving the plates in and out of the incubator when the medium is changed, or even when closing the incubator door. The precise volume of 190 µL of medium per well is crucial to keep the DRG explants in place during the first day of culture. Loose DRG explants can be identified easily during the daily control, as they swim in the medium instead of adhering to the coverslip.
3. Coculture
- Transfer of Schwann cells to the DRG explant culture
- Prepare the coculture medium by adding 0.1% ascorbic acid to the DRG growth medium.
- On day 3 of the DRG explant culture, carefully replace the DRG growth medium with 250 µL of the coculture medium containing 30,000 Schwann cells (from step 1.8) per well.
- Keep the coculture of the DRG explants and Schwann cells for up to 22 days. Replace 250 µL of the coculture medium carefully every other day, and observe the appearance of the cells using a microscope.
NOTE: For an example of DRG axons and Schwann cells in the coculture in the first days of culture, see Figure 2.
Representative Results
Figure 1: Stages of Schwann cell culture. Exemplary brightfield pictures of cultured Schwann cells (A) before and (B) after magnetic cell separation. Before magnetic cell separation, the culture includes Schwann cells with an elongated and spindle shape morphology, flat and spread-out appearing fibroblasts, and remnants of connective tissue. To achieve a purer culture of Schwann cells, magnetic cell separation is performed. Scale bars: 200 µm.
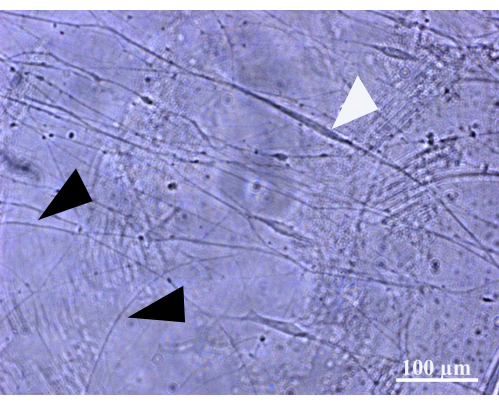
Figure 2: Appearance of Schwann cells and axons in the coculture. Exemplary picture of the coculture on day 3. Black arrows show thin DRG axons, and the white arrow points to a Schwann cell with an elongated and spindle-shaped morphology attached to an axon. Scale bar: 100 µm.
Declarações
The authors have nothing to disclose.
Materials
| Ascorbic acid | Sigma Aldrich GmbH, Steinheim, Germany | A4403-100MG | |
| B27-supplement | Thermo Fisher Scientific, Schwerte, Germany | 17504-044 | |
| Biosphere Filter Tip, 100 µL | Sarstedt, Nümbrecht, Germany | 70760212 | |
| Biosphere Filter Tip, 1250 µL | Sarstedt, Nümbrecht, Germany | 701186210 | |
| Biosphere Filter Tip, 20 µL | Sarstedt, Nümbrecht, Germany | 701114210 | |
| Biosphere Filter Tip, 300 µL | Sarstedt, Nümbrecht, Germany | 70765210 | |
| Bovine serum albumin | Carl Roth, Karlsruhe, Germany | 8076.4 | |
| Cell strainer, 100 µM | BD Bioscience, Heidelberg, Germany | 352360 | |
| Centrifuge 5810-R | Eppendorf AG, Hamburg, Germany | 5811000015 | |
| CO2 Incubator Heracell | Heraeus Instruments, Hanau, Germany | 51017865 | |
| Coverslips 12 mm | Carl Roth, Karlsruhe, Germany | P231.1 | |
| Curved fine forceps | Fine Science Tools GmbH, Heidelberg, Germany | 11370-42 | |
| Dispase II | Sigma Aldrich GmbH, Steinheim, Germany | 4942078001 | |
| Distilled water (Water Purification System) | Millipore, Molsheim, France | ZLXS5010Y | |
| DMEM/F-12, GlutaMAX | Thermo Fisher Scientific, Schwerte, Germany | 31331093 | |
| DPBS (no Ca2+ and no Mg2+) | Sigma Aldrich GmbH, Steinheim, Germany | D8537-6X500ML | |
| Ethanol | VWR, Radnor, USA | 1009862500 | |
| FCS | Sigma Aldrich GmbH, Steinheim, Germany | F7524 | FCS must be tested for Schwann cell culture |
| Fine forceps (Dumont #5) | Fine Science Tools GmbH, Heidelberg, Germany | 11252-20 | |
| Forceps | Fine Science Tools GmbH, Heidelberg, Germany | 11370-40 | |
| Forskolin | Sigma Aldrich GmbH, Steinheim, Germany | F6886-10MG | |
| Gentamycin | Thermo Fisher Scientific, Schwerte, Germany | 5710064 | |
| HBSS (no Ca2+ and no Mg2+) | Thermo Fisher Scientific, Schwerte, Germany | 14170138 | |
| HERAcell Incubator | Heraeus Instruments, Hanau, Germany | 51017865 | |
| Heraguard ECO 1.2 | Thermo Fisher Scientific, Schwerte, Germany | 51029882 | |
| Horse serum | Pan-Biotech, Aidenbach, Germany | P30-0712 | |
| Laminin | Sigma Aldrich GmbH, Steinheim, Germany | L2020-1MG | |
| Leibovitz´s L-15 Medium | Thermo Fisher Scientific, Schwerte, Germany | 11415064 | |
| L-Glutamine 200 mM | Thermo Fisher Scientific, Schwerte, Germany | 25030024 | |
| MACS Multistand | Miltenyi Biotec, Bergisch Gladbach, Germany | 130042303 | |
| Microscissors | Fine Science Tools GmbH, Heidelberg, Germany | 15000-08 | |
| Microscope | Motic, Wetzlar, Germany | Motic BA 400 | |
| Microscope Axio observer 7 | Zeiss, Oberkochen, Germany | 491917-0001-000 | |
| Microscope slide | VWR, Radnor, USA | 630-1985 | |
| MiniMACS separator | Miltenyi Biotec, Bergisch Gladbach, Germany | 130091632 | |
| MS columns | Miltenyi Biotec, Bergisch Gladbach, Germany | 130-042-201 | |
| Neubauer counting chamber | Assistant, Erlangen, Germany | 40441 | |
| Neuregulin | Peprotech, Rocky Hill, USA | 100-03 | |
| Neurobasal medium | Thermo Fisher Scientific, Schwerte, Germany | 21103049 | |
| NGF | Sigma Aldrich GmbH, Steinheim, Germany | N1408 | |
| Normal goat serum | Biozol, Eching, Germany | S-1000 | |
| Nunclon multidishes, 4 well | Sigma Aldrich GmbH, Steinheim, Germany | D6789 | |
| Paraformaldehyde | Acros Organics, New Jersey, USA | 10342243 | |
| Penicillin/Streptomycin | Thermo Fisher Scientific, Schwerte, Germany | 15140-122 | |
| Pipetboy | Eppendorf AG, Hamburg, Germany | 4430000018 | |
| Pipettes | Eppendorf AG, Hamburg, Germany | 2231300004 | |
| Poly-D-Lysin | Sigma Aldrich GmbH, Steinheim, Germany | P6407-5MG | |
| Poly-L-Lysin | Sigma Aldrich GmbH, Steinheim, Germany | P4707-50ML | |
| Reaction tubes, 15 mL | Sarstedt, Nümbrecht, Germany | 62554502 | |
| Reaction tubes, 50 mL | Sarstedt, Nümbrecht, Germany | 62547254 | |
| Reaction vessels, 1.5 mL | Sarstedt, Nümbrecht, Germany | 72690001 | |
| Safety Cabinet S2020 1.8 | Thermo Fisher Scientific, Schwerte, Germany | 51026640 | |
| Scissors | Fine Science Tools GmbH, Heidelberg, Germany | 14083-08 | |
| Serological pipette, 10 mL | Sarstedt, Nümbrecht, Germany | 861254025 | |
| Serological pipette, 25 mL | Sarstedt, Nümbrecht, Germany | 861685001 | |
| Serological pipette, 5 mL | Sarstedt, Nümbrecht, Germany | 861253001 | |
| Spatula | Fine Science Tools GmbH, Heidelberg, Germany | 10094-13 | |
| Stereomicroscope Discovery.V8 | Zeiss, Oberkochen, Germany | 495015-0012-000 | |
| Surgical scissors | Fine Science Tools GmbH, Heidelberg, Germany | 14007-14 | |
| TC dish 100, cell + | Sarstedt, Nümbrecht, Germany | 833902300 | |
| TC dish 35, cell + | Sarstedt, Nümbrecht, Germany | 833900300 | |
| TC dish 60, cell + | Sarstedt, Nümbrecht, Germany | 833901300 | |
| Thy-1 Microbeads (MACS Kit) | Miltenyi Biotec, Bergisch Gladbach, Germany | 130-094-523 | |
| Trypan Blue Solution 0.4% | Thermo Fisher Scientific, Schwerte, Germany | 15250061 | |
| Trypsin (2.5%), no phenol red | Thermo Fisher Scientific, Schwerte, Germany | 15090-046 | |
| Trypsin-EDTA (0.05%), phenol red | Thermo Fisher Scientific, Schwerte, Germany | 25300-054 | |
| Type I Collagenase | Sigma Aldrich GmbH, Steinheim, Germany | C1639 | |
| Water bath type 1008 | GFL, Burgwedel, Germany | 4285 |

