Isolation of Neural Stem Progenitor Cells from the Spinal Cord Periventricular Region of an Adult Rat
Abstract
Source: Mothe, A., et al., Isolation of Neural Stem/Progenitor Cells from the Periventricular Region of the Adult Rat and Human Spinal Cord. J. Vis. Exp. (2015).
This video presents a method for isolating neural stem/progenitor cells from the periventricular tissue of the spinal cord in adult rats. The technique includes incubating the tissue fragments in a solution containing proteolytic enzymes to disrupt the extracellular matrix and release the cells. Subsequently, the released cells are plated in a medium containing specific growth factors that enhance neural stem/progenitor proliferation and promote aggregation into three-dimensional neurospheres.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Preparation of Dissection Buffers and Culture Media
- For the isolation of rat spinal cord, prepare 100 ml dissection buffer (1x PBS + 0.6% glucose + 2% penicillin-streptomycin) and refrigerate.
- Prepare 100 ml serum-free medium (SFM) and warm at 37 °C. To prepare SFM, add 2 mM L-glutamine, 100 μg/ml penicillin-streptomycin, 2% B27, and 10% hormone mix to Neurobasal-A medium. To prepare hormone mix, make a 1:1 DMEM/F12 medium containing 0.6% glucose, 3 mM NaHCO3, 5 mM HEPES, 25 μg/ml insulin, 100 μg/ml apo-transferrin, 10 μM putrescine, 30 nM selenium, and 20 nM progesterone.
- Prepare the EFH growth factor-enriched plating media (to SFM add 20 ng/ml EGF, 20 ng/ml bFGF, and 2 μg/ml heparin) and warm at 37 °C.
2. Harvesting and Dissection of Adult Rat Periventricular Spinal Cord Tissue
- Sterilize dissecting instruments in ethanol and rinse in sterile saline, or autoclave instruments in advance. Give rats (6 – 8 weeks old) a lethal injection of sodium pentobarbital (1 cc at 65 mg/ml) or an overdose of anesthetic (i.e., 4% isoflurane) according to one’s institution-approved animal protocol. Douse the back of the rat with 70% ethanol and remove the skin on the dorsal surface with large dissection scissors.
- Holding the scissors perpendicular to the dorsal surface, transversely cut the vertebral column above the hindlimbs. Use smaller scissors to longitudinally cut the dorsal muscle overlying the vertebral column in a rostral direction to expose the spinous processes.
- Starting at the exposed caudal end, insert rongeurs or a small blunt bone-cutting instrument extradurally into the lateral aspect of the spinal canal in the space between the spinal cord and the vertebral column. Make small cuts into the lamina (bony arches left and right of the spinous processes on each side of the vertebrae) and carefully peel away the lamina to expose the spinal cord (Figure 1A-D). Ensure the angle of the blades is shallow and parallel to the cord so the underlying spinal cord is not damaged.
- Continue laminectomy in the rostral direction to expose the thoracic and cervical spinal cord.
- Gently excise the spinal cord from the vertebral column with blunt tissue forceps and use microscissors to carefully sever the roots to release the cord. Place the excised spinal cord in a Petri dish containing 4 °C sterile rat dissection buffer (described above in 1.1). Rinse the tissue in freshly prepared 4 °C dissecting buffer and cut the spinal cord transversely into 1 cm segments using scissors (Figure 1E).
- For each segment of tissue, use one hand to hold the tissue with fine forceps. With the other hand, use microscissors to carefully remove the overlying meninges, white matter, and most of the grey matter with the aid of a dissecting scope. Alternatively, use fine forceps (Dumont #4) to peel away most of the grey and white matter, leaving only the periventricular region of the spinal cord which includes the ependyma and a small amount of surrounding grey matter (Figure 1F-H).
- Pool the dissected periventricular tissue into a 10 cm sterile Petri dish containing cold rat dissection buffer.
3. Isolation and Culturing of Adult Rat Spinal Cord NSPCs
- Perform the following steps aseptically in a laminar flow hood.
- Mince the dissected rat periventricular tissue into 1 mm pieces with microscissors.
- Enzymatically dissociate the minced tissue with proteolytic enzymes using the papain dissociation kit as indicated in the table of materials/reagents.
NOTE: The reagent components include four vials: Vial 1: Earle's Balanced Salt Solution (EBSS). Vial 2: Papain containing L-cysteine and EDTA. Vial 3: Deoxyribonuclease I (DNase). Vial 4: Ovomucoid protease inhibitor with bovine serum albumin (BSA).
NOTE: Papain is a sulfhydryl protease with wide specificity for protein substrates. The papain herein is derived from the latex of the Carica papaya plant and degrades most protein substrates more extensively than pancreatic proteases. During the dissociation process, there is some cell damage causing DNA to be released into the dissociation medium, which will increase viscosity and make pipetting difficult. Thus, DNase is included in the cell isolation procedure to digest the DNA without damaging the intact cells. Ovomucoids are glycoprotein protease inhibitors used to inhibit papain activity after the dissociation step.
- At first use, reconstitute the albumin ovomucoid inhibitor mixture (vial 4) with 32 ml of EBSS (vial 1), and then store at 4 °C and use for subsequent isolations. NOTE: This yields a solution at an effective concentration of 10 mg of ovomucoid inhibitor and 10 mg of albumin per ml.
- Add 5 ml of EBSS (vial 1) to the papain vial (vial 2), yielding a solution at 20 units of papain/ml in 1 mM L-cysteine with 0.5 mM EDTA. Check that the papain solution appears clear when completely dissolved. Otherwise, place the vial in a 37 °C water bath for ten minutes until the papain is completely dissolved to ensure the total activity of the enzyme.
- Add 500 μl of EBSS (vial 1) to the DNase vial (vial 3) and mix gently as DNase is sensitive to shear denaturation. Add 250 μl of the DNase solution to the papain vial, resulting in a final concentration of approximately 20 units/ml papain and 0.005% DNase. Save the remaining DNAse for use later.
- Place the minced rat tissue in the papain solution.
- Incubate at 37 °C with constant agitation on a rocker platform to dissociate the tissue in the activated papain solution. Incubate rat tissue for 45 min to 1 hr.
- Triturate the mixture with a 10 ml pipette to dissociate any remaining tissue pieces to yield a cloudy cell suspension. Transfer the cell suspension (do not include any pieces of undissociated tissue) into a sterile 15 ml conical tube and centrifuge at 300 x g for 5 min at RT.
- Resuspend the pelleted cells in medium containing ovomucoid, a papain inhibitor.
- Prepare the ovomucoid solution by mixing 2.7 ml EBSS (vial 1) with 300 μl of the reconstituted albumin-ovomucoid inhibitor solution (vial 4) in a 15 ml conical tube. Add 150 μl of DNase solution (vial 3).
- Discard the supernatant from the pelleted cells and immediately resuspend the cell pellet in the diluted DNase albumin-inhibitor mixture.
- Separate intact cells from cell membranes by centrifugation through a single step discontinuous density gradient.
- Prepare the discontinuous density gradient by adding 5 ml of albumin-inhibitor solution (vial 4) to a 15 ml tube, and using a 5 ml pipette, gently and slowly layer the cell suspension (prepared as described above) on top of the albumin-inhibitor solution.
- Centrifuge at 70 x g for 6 min at RT.
NOTE: The interface between the two layers of the gradient should be clearly visible, although minimal mixing at this boundary does not affect the result. Membrane fragments remain at the interface and dissociated cells pellet at the bottom of the tube.
- Discard the supernatant and resuspend the cell pellet in 1 ml of rat EFH medium (pre-warmed at 37 °C). Count live cell density with a hemocytometer and plate the cells into a T25 culture flask at a density of 10 cells/μl in EFH. The typical yield of cells from rat tissue is about 2 x 106 cells with about 80% viability. If clonal cultures are desired, seed cells at a density of less than 10 cells/μl. Incubate the flasks at 37 °C in 5% CO2 and allow the cultures to grow undisturbed for 1 week to avoid aggregation of spheres.
Representative Results
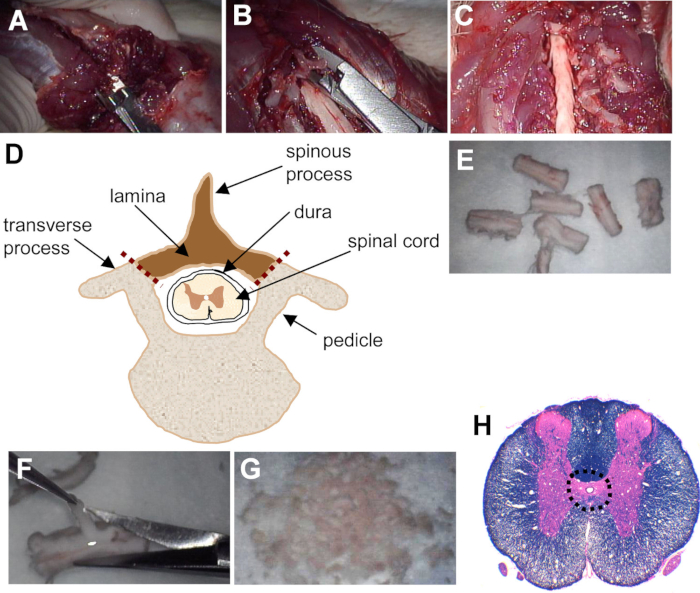
Figure 1. Laminectomy of rat spinal cord and dissection of periventricular region. (A) At the exposed caudal end of the spinal cord, rongeurs are inserted extradurally into the lateral aspect of the spinal canal. (B) Small cuts are made into the lamina on each side of the vertebrae and the lamina is carefully peeled away to expose the spinal cord. (C) The exposed cervical spinal cord after laminectomy. (D) Schematic of thoracic vertebral cross-section showing spinal cord and location of cuts made for laminectomy (depicted with red dotted lines). The laminae and spinous process (shaded region) are removed. (E) The spinal cord is cut transversely into 1 cm segments. (F) The overlying meninges, white matter, and most of the grey matter is carefully removed using microscissors. (G) Minced periventricular tissue. (H) Transverse section of rat spinal cord stained with luxol fast blue and hematoxylin and eosin with dotted outline showing dissected periventricular region.
Declarações
The authors have nothing to disclose.
Materials
| 1x PBS | Life Technologies | #10010023 | Dissection buffer |
| 1x HBSS | Life Technologies | #14175095 | Dissection buffer |
| D-glucose | Sigma | # G-6152 | Prepare 30% glucose stock solution for dissection buffer and hormone mix |
| Penicillin-Streptomycin | Life Technologies | #15140-148 | Dissection buffer and culture medium |
| Neurobasal-A | Life Technologies | #10888-022 | Culture medium |
| L-glutamine, 200 mM | Life Technologies | #25030-081 | Culture medium |
| B27 | Life Technologies | #12587010 | Culture medium |
| DMEM | Life Technologies | #11885084 | Hormone mix |
| F12 | Life Technologies | #21700-075 | Hormone mix |
| NaHCO3 | Sigma | # S-5761 | Prepare 7.5% NaHCO3 stock solution for hormone mix |
| HEPES | Sigma | #H9136 | Prepare 1M HEPES stock solution for hormone mix |
| Insulin | Sigma | #I-5500 | Hormone mix |
| Apo-transferrin | Sigma | #T-2252 | Hormone mix |
| Putrescine | Sigma | # P7505 | Hormone mix |
| Selenium | Sigma | #S-9133 | Hormone mix |
| Progesterone | Sigma | #P-6149 | Hormone mix |
| EGF, mouse | Sigma | #E4127 | Prepare 100 μg/ml stocks in B27 and aliquot; EFH medium |
| bFGF, human recombinant | Sigma | #F0291 | Prepare 100 μg/ml stocks in B27 and aliquot; EFH medium |
| Heparin, 10,000 U | Sigma | #H3149 | Prepare 27.3 mg/ml stocks in hormone mix and aliquot; EFH medium |
| Papain dissociation kit | Worthington Biochemicals | #LK003150 | Contains EBSS, papain, DNase, ovomucoid protease inhibitor with BSA |
| Sodium Pentobarbital | Bimeda – MTC Animal Health Inc | DIN 00141704 | |
| Tissue Forceps: Addisons | Fine Science Tools | #11006-12 | Serrated standard tip; micro-tip also available |
| Fine Forceps: Dumont #4 | Fine Science Tools | #11241-30 | |
| Microscissors | Fine Science Tools | #15024-10 | Round-handled Vannas |
| Rongeurs | Bausch & Lomb | N1430 | |
| 10 mm Petri dishes | Nunc | 1501 | |
| T25 Culture flasks | Nunc | 156367 | |
| 6 well plates | Nunc | CA73520-906 |

