Generating Schwann Cell-Like Cells from Bone Marrow Stromal Cells
Abstract
Source: Tsui, Y., et al. Hypoxic Preconditioning of Marrow-derived Progenitor Cells As a Source for the Generation of Mature Schwann Cells. J. Vis. Exp. (2017)
This video demonstrates a method for obtaining Schwann cell-like cells from multipotent bone marrow stromal cells (BMSCs). Initially, the BMSCs undergo hypoxia treatment, followed by directed differentiation into neuronal progenitor cells. Subsequently, specific media and culture conditions are employed to induce the transformation of these progenitor cells into Schwann cell-like cells.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Preparation of Rat MSC Cultures
- Harvest of MSCs from the femur
- Autoclave all dissection tools (i.e., fine dissecting scissors, blunt-tipped cutting scissors, and toothed forceps) at 180 °C for at least 2 h before use.
- Prepare MSC growth medium comprising of minimal essential medium – alpha modification (αMEM) supplemented with 15% fetal bovine serum (FBS) and penicillin/streptomycin (P/S, 1% v/v).
- Sacrifice young male Sprague Dawley rats (200-250 g body weight) by pentobarbitone overdose (240 mg/kg body weight, intraperitoneal).
NOTE: Marrow samples from different rats should be processed separately. - Place the sacrificed animals in the supine position. Clean their abdomen and lower limbs thoroughly with 70% ethanol.
- Remove the skin and subcutaneous tissue over the medial thighs using fine dissecting scissors and forceps. Remove the thigh muscles circumferentially until the femur is exposed. Continue this proximally and distally until the knee and hip joints are seen. Disarticulate the femur through the hip and knee joint using blunt-tipped cutting scissors.
NOTE: Do not transect the femur to expose the marrow cavity at this stage. Transfer intact femurs to a laminar flow tissue culture hood for further processing. - Use blunt-tipped cutting scissors to transect the distal and proximal ends of the femur through the metaphysis.
- Place a 70 μm cell strainer over a 50 mL conical tube. Insert a 21 G, 10 mL syringe containing phosphate-buffered saline (PBS, 10 mM Na2HPO4, pH 7.4) into the exposed femoral canal and flush the marrow content into the conical tube by repeated flushing.
NOTE: Approximately 20 mL of PBS is used to flush each femur. If the color of the flushed content remains blood-stained and turbid, a larger volume can be used. - Collect the cells by centrifugation at 480 x g for 5 min. Discard the supernatant. Resuspend the cell pellet in 10 mL of MSC growth medium. Plate the cells onto a 10 cm tissue culture dish. Place the tissue culture dishes into a cell incubator (37 °C, 5% CO2). Record the initial day of plating as day 0.
NOTE: In all steps involving centrifugation, set the brake for maximal deceleration.
- Establishment and expansion of MSC colonies
NOTE: This protocol relies on tissue culture plastic adherence to select MSCs from within the marrow cavity. The bone marrow content is allowed to adhere to the tissue culture plastic for 2 days.- On day 2, rinse the culture plates thrice with 10 mL of PBS to remove non-adherent cells. Replace the PBS with 10 mL of MSC growth medium after rinsing. Wash the cells with PBS and replenish them with MSC growth medium every 3 days.
NOTE: MSC colonies should be visible by day 6-7 (Figure 1A). - Passage the cells by day 10 by removing the growth medium and rinsing the cells with PBS. Add 1.5 mL of recombinant enzymatic cell dissociation reagent and incubate at 37 °C for 5 min. Add 3 mL of MSC growth medium to neutralize the reaction. Collect the detached cells by centrifugation at 250 x g for 5 min.
- Quantify the cells within the pellet using a hemocytometer after an appropriate dilution in PBS.
- Seed passaged cells at a density of 40,000 cells/cm2 into a 10-cm culture plate in an MSC growth medium.
NOTE: Rat MSCs should reach 80-90% confluence within 2 days of passaging (Figure 1B). Cells can be passaged as described in step 1.2.2 for up to 8 passages. MSCs can be characterized using immunocytochemistry and their capacity for trilineage differentiation (Figure 2). Only MSC cultures between passages 3 and 8 are subject to subsequent hypoxic preconditioning and neural progenitor enrichment. MSCs of greater passage number adopt a flattened morphology (Figure 1C) and do not produce sufficient neural progenitors. These cultures should be discarded.
- On day 2, rinse the culture plates thrice with 10 mL of PBS to remove non-adherent cells. Replace the PBS with 10 mL of MSC growth medium after rinsing. Wash the cells with PBS and replenish them with MSC growth medium every 3 days.
2. Preparation of Human BMSC Cultures
- Dilute 1 mL of human bone marrow aspirate with 9 mL of MSC growth medium and plate the cells on a 10-cm tissue culture dish. Maintain the cultures in a cell incubator (37 °C, 5% CO2).
- Remove the medium after 2 days and gently rinse the cultures thrice with 10 mL of PBS to remove non-adherent cells. After the final rinse, remove the PBS and replace it with 10 mL of MSC growth medium. Replenish the growth medium after every 3 days of culture after the PBS rinse.
NOTE: MSC colonies should be visible by day 6-7. The number of colonies may vary between subjects. - Passage the cells on day 10, as described in step 1.2.2. Quantify the cells within the pellet using a hemocytometer after an appropriate dilution in PBS. Seed the passaged cells at 40,000 cells/cm2 density onto a 10 cm culture plate in an MSC growth medium.
NOTE: Human MSCs (Figure 1D) demonstrate a similar morphology to rat MSCs and should reach 80-90% confluence within 2 days of passaging. They should be characterized using immunocytochemistry and their capacity for trilineage differentiation. As with rat MSCs, human MSCs between passages 3 and 8 are subject to subsequent hypoxic preconditioning and neural progenitor enrichment.
3. Hypoxic Preconditioning
- Disassemble the hypoxia chamber components (i.e., base, lid, trays) after releasing the ring clamp and clean the individual parts with 70% ethanol. Place the chamber components within a laminar flow tissue culture hood for sterilization under UV light for 15 min.
- Before hypoxic preconditioning, remove the medium and rinse the rat and human MSC cultures (sections 1 and 2) with 10 mL of PBS. Replace the PBS with 10 mL of MSC growth medium supplemented with 25 mM HEPES.
NOTE: The MSCs cultured upon 10 cm dishes should have reached 80-90% confluency before being subject to hypoxic preconditioning. - Place the culture dishes within the hypoxia chamber. Reassemble the chamber components and tighten the ring clamp. Flush a gas mixture of 99% N2/1% O2 into the chamber at a flow rate of 10 L/min for 5 min.
- Seal the connecting ends of the hypoxia chamber to ensure no gas leakage. Place the chamber inside the cell incubator (37 °C, 5% CO2) for 16 h.
- Upon completion of the hypoxic preconditioning, remove the cultures from the chamber in preparation for the subsequent neural progenitor enrichment culture.
4. Neural Progenitor Enrichment Culture
- Prepare neural progenitor medium comprised of Dulbecco's modified eagle medium/Ham's nutrient mixture F12 (DMEM/F12) supplemented with B27 (2% v/v), basic fibroblast growth factor (bFGF, 20 ng/mL), epidermal growth factor (EGF, 20 ng/mL), and P/S (1% v/v).
- Detach the hypoxic preconditioned rat/human MSCs, as described in step 1.2.2. Collect the detached cells by centrifugation at 250 x g for 5 min. Quantify the cells within the pellet using a hemocytometer after appropriate dilution in PBS.
- Resuspend the cells in neural progenitor medium and seed on low-attachment, 6-well plates at a density of 6,000 cells/cm2. Place the culture in a cell incubator (37 °C, 5% CO2) for 12 days. Replenish 75% of the neural progenitor medium every 3 days.
NOTE: Sizeable non-adherent cell clusters should be observed by days 6-7. By day 10-12, neurospheres with a diameter ≥ 100 μm can be observed (Figure 3). Hypoxic preconditioned MSCs should yield more neurospheres than MSCs cultured under normoxic conditions. - Collect neurospheres on day 12 by aspirating them into a 10-mL pipette and transferring them to a 15 mL conical tube. Centrifuge the neurospheres at 250 x g for 5 min.
NOTE: Neurospheres can be characterized on day 12 for neural progenitor markers, such as nestin and GFAP.
5. Generation of Schwann cell-like cells
- Prepare glial induction medium comprised of αMEM supplemented with β-Heregulin (100 ng/mL), bFGF (10 ng/ml), platelet-derived growth factor (PDGF-AA, 5 ng/mL), FBS (10%), and P/S (1% v/v).
- Plate the neurospheres prepared in section 4 in PDL/laminin-coated 6-well plates at a density of 5-10 spheres per cm2 in 1.5 mL of glial induction medium per well. Replace the glial induction medium every 2 days after rinsing the cells with PBS.
NOTE: Cells from seeded neurospheres are seen to migrate outwards by day 2. By day 7, migratory cells have a tapered appearance and should demonstrate immunopositivity for the Schwann cell markers p75 neurotrophin receptor (p75) and S100β. These cells are referred to as SCLCs.
Representative Results
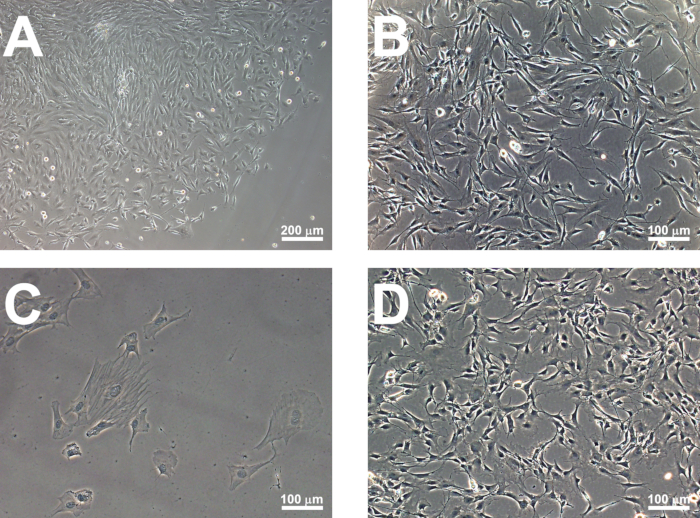
Figure 1: Establishment of MSC colonies. Sizeable MSC colonies should be visible 6-7 days after the plating of bone marrow cells onto tissue culture plastic. A representative image of a rat MSC colony is shown (A), while human colonies demonstrate a similar appearance. Colonies can be passaged on day 10. Seen at higher magnification, both rat (B) and human (D) MSCs exhibit a characteristic fibroblast-like morphology after passaging. MSCs maintained for high passage numbers acquire a flattened, quadrangular morphology (C) and should be discarded.
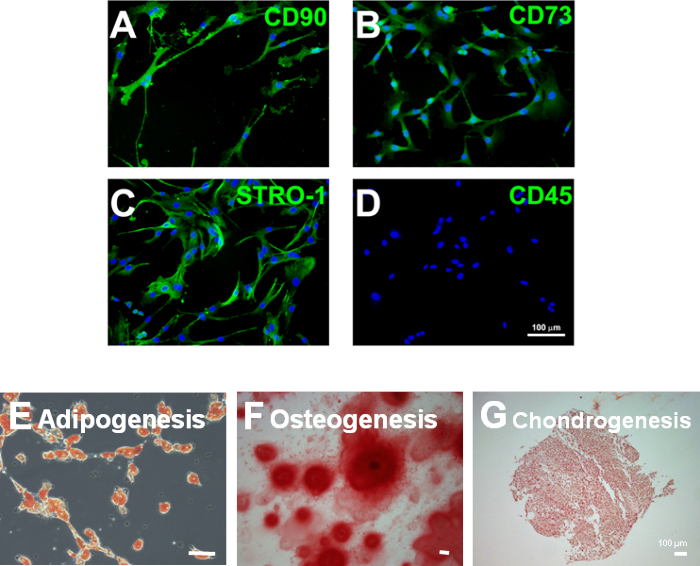
Figure 2: Characterization of MSCs. (A–D) Representative images of human MSCs. MSCs can be characterized by the expression of appropriate markers, such as CD90 (A), CD73 (B), and Stro-1 (C), and by the absence of hematopoietic stem cell markers, such as CD45 (D). (E–G) Rat MSCs isolated and expanded as described in the protocol demonstrate multipotency in their ability to form adipocytes (E; fat deposits stained with Sudan Red), osteoblasts (F; pericellular matrix stained with Alizarin Red), and chondrocytes (G; proteoglycans stained with Safranin-O) under appropriate culture conditions. Scale bars = 100 μm.
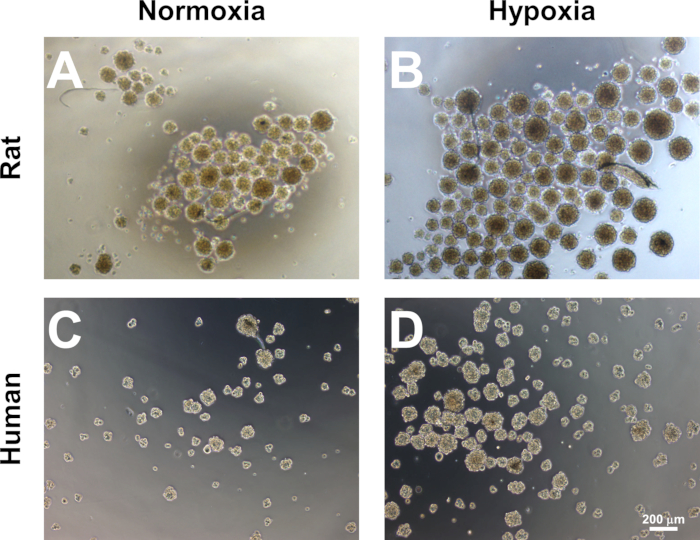
Figure 3: Enrichment of neural progenitors from MSCs. Both rat (A) and human (C) MSCs form neurospheres when cultured upon low-adherence tissue culture plastic in a medium supplemented with EGF/bFGF. The numbers and average diameter of rat (B) and human (D) spheres are enhanced via the hypoxic preconditioning of MSCs (16 h, 1% O2) before sphere induction. Scale bars = 200 μm.
Declarações
The authors have nothing to disclose.
Materials
| αMEM | Sigmaaldrich | M4526 | |
| DMEM/F12 | Thermofisher scientific | 12400-024 | |
| FBS | Biosera | FB-1280/500 | |
| B27 | Thermofisher scientific | 17504-001 | |
| Epidermal growth factor (EGF) | Thermofisher scientific | PHG0313 | |
| Basic fibroblast growth factor (bFGF) | Peprotech | 100-18B/100UG | |
| Platelet-derived growth factor-AA (PDGF-AA) | Peprotech | 100-13A | |
| Heregulin beta-3, EGF domain (β-Her) | Millipore | 01-201 | |
| Poly-D-lysine (PDL) | Sigmaaldrich | P7886-1G | |
| Laminin | Thermofisher scientific | 23017015 | |
| Penicillin / streptomycin (P/S) | Thermofisher Scientific | 15140-122 | |
| TrypLE Express | Thermofisher Scientific | 12604-013 | |
| 10 cm plate for adherent culture | TPP | 93100 | Used for selection of MSCs |
| 6-well plate for adherent culture | TPP | 92006 | Used for expansion of MSCs following passaging |
| UltraLow 6-well plate for nonadherent culture | Corning | 3471 | Used for neural progenitor enrichment |
| anti-human CD90(Thy-1) | BD Biosciences | 555593 | |
| anti-human CD73 | BD Biosciences | 550256 | |
| anti-human/rat STRO-1 | R&D Systems | MAB1038 | |
| anti-human CD45 | BD Biosciences | 555480 | |
| Hypoxia chamber | Billups-Rothenberg | MIC-101 | |
| HEPES buffer | Sigmaaldrich | H4034-100G | |
| anti-human nestin | R&D Systems | MAB1259 | |
| anti-rat CD90(Thy-1) | BD Biosciences | 554895 | |
| anti-rat CD73 | BD Biosciences | 551123 | |
| anti-rat nestin | BD Biosciences | MAB1259 | |
| anti-rat CD45 | BD Biosciences | 554875 | |
| Anti-S100β | Dako | Z031101 | |
| Anti-p75 | Millipore | MAB5386 | |
| Anti-GFAP | Sigmaaldrich | G3893 | |
| Anti-Class III-beta tubulin (Tuj-1) | Covance | MMS-435P | |
| Anti-Human nuclei | Millipore | MAB1281 |

