pH Modulation Assay on Supported Lipid Bilayers to Detect Protein-Phosphoinositide Interactions
Abstract
Source: Shengjuler, D., et al., PIP-on-a-chip: A Label-free Study of Protein-phosphoinositide Interactions. J. Vis. Exp. (2017)
In this video, we demonstrate protein-phosphoinositide interactions in a microfluidic pattern using the pH modulation assay, using pH-sensitive fluorescent dye tagged to phosphatidylethanolamine lipid of a supported lipid bilayer, called a microfluidic platform. The modulations in pH after protein binding to phospholipid cause fluorescence quenching.
Protocol
1. Forming Supported Lipid Bilayers (SLBs)
- Transfer 100 µL of PI(4,5)P2-containing SUVs into a 0.65 mL microcentrifuge tube. Adjust the pH of the solution to ~3.2 by adding 6.4 µL of 0.2 N hydrochloric acid.
NOTE: Confirm the pH of the solution with a pH meter equipped with a micro pH probe (see table of materials). - Pipette 10 µL of the pH-adjusted SUV solution into each channel through the inlet and apply pressure through the pipette until the solution reaches the outlet. Detach the tip from the pipette and leave it attached to the device.
- Repeat the above step for each channel and then incubate the device for 10 min at RT.
NOTE: Injection of vesicles into microchannels should be performed immediately after the device assembly. - Cut sets of inlet and outlet tubing each 60 cm (inlet tubing) and 8 cm (outlet tubing) long, respectively.
NOTE: Cutting the tubing diagonally and creating a sharp edge, makes it easier to insert the tubing into the inlets and outlets. The outlet tubing should have an arc shape. The length of the inlet tubing may vary based on the microscope setup. The internal diameter of the tubing is 0.05 cm. - Using tweezers, connect the outlet tubing set to the device, and then tape the device onto a microscope stage.
- Submerge one end of the inlet tubing set in 25 mL of running buffer contained in a conical tube and tape it to make sure that the tubing is secured.
- Place the conical tube on a higher ground (~20 cm) than the device in order to push the solution through the microchannels via gravity flow; a lab jack can be used to achieve this.
- For each inlet tube, use a syringe to draw 1 mL of running buffer from the free end of the tubing. Remove the pipette tip from the inlet and insert the free end of the inlet tubing into the device.
NOTE: Introduce a drop of running buffer onto the inlet to reduce the probability of an air bubble being introduced into the channel during this step. After the inlet tubing is attached to the device, use a lint-free wipe to remove the excess buffer. - Repeat the above step to connect all the inlet tubing pieces to the device.
NOTE: Flowing running buffer through the channels helps to remove excess vesicles and equilibrate the bilayer to experimental conditions. - Open the microscope control software (see table of materials). On the left panel, click on the "Microscope" tab and choose the "10X" objective.
- Click on "Live" and then "Alexa 568" image icons on the toolbar. Using the fine and course adjustment knobs, focus on the microchannels.
- Scan through the device to check the quality of the SLBs and the channels (Figure 1). Then, click "FL Shutter Closed" image icon on the toolbar.
- Click on the "Acquisition" tab, and under "Basic adjustments" select "Exposure time." Set the exposure time to "200 ms".
- On the left panel, click on "Multidimensional Acquisition" and under the filters menu select the red channel (marked as "Alexa 568"). Then, click on the "time lapse" menu, set the time interval to 5 min, duration to 30 min, and click "Start".
NOTE: Based on the duration (30 min) and the flow rate (~1.0 µL/min), 30 µL of running buffer is used to equilibrate the SLB within a channel, i.e. 240 µL of running buffer is used for all 8 channels. - Select the "Circle" tool under the "Measure" tab and draw a circle in any channel. Right click while the circle is selected and choose "Properties". Under the "Profile" tab, check "all T" to view the fluorescence intensity as a function of time.
- Make sure this curve reaches a plateau, which indicates equilibrium, before proceeding to the next step.
- Lower the buffer solution to an equal ground as the device to stop the flow.
- Save the time lapse file.
2. Testing PLC-δ1 PH domain interaction with PI(4,5)P2-containing SLBs
- Prepare dilutions of the PH domain using the running buffer as a diluent.
NOTE: Since there are eight channels within the device, use at least two of them for blank controls. Pick the far ends on each side and use the remaining channels for protein dilutions. The following PH domain concentrations were tested: 0.10, 0.25, 0.50, 1.00, and 2.50 µM. Approximately 200 µL of each dilution was sufficient to reach equilibrium within 30 min. - One at a time, detach each outlet tubing and apply 200 µL of each protein dilution into the outlet channel using a pipette. Do not apply any pressure, let gravity do the work. Detach the tip from the pipette and leave it attached to the microfluidic device.
- Repeat the above step for each channel and make sure that air bubbles are not introduced into the channels during this process.
- Lower the inlet tubing to a ground below the microfluidic device to start flowing the protein through the microchannels. Tape the free end of the tubing to a waste container. Flow the protein dilutions for 30 min.
NOTE: This will reverse the flow and allow the protein to be introduced into the channels. The time to equilibration and volume of protein dilutions needed will be dependent on the affinity of the interaction. - On the left panel of the software, under the "Time Lapse" tab, click on "Start" to begin imaging again.
- When the experiment is complete, save the time lapse file.
Representative Results
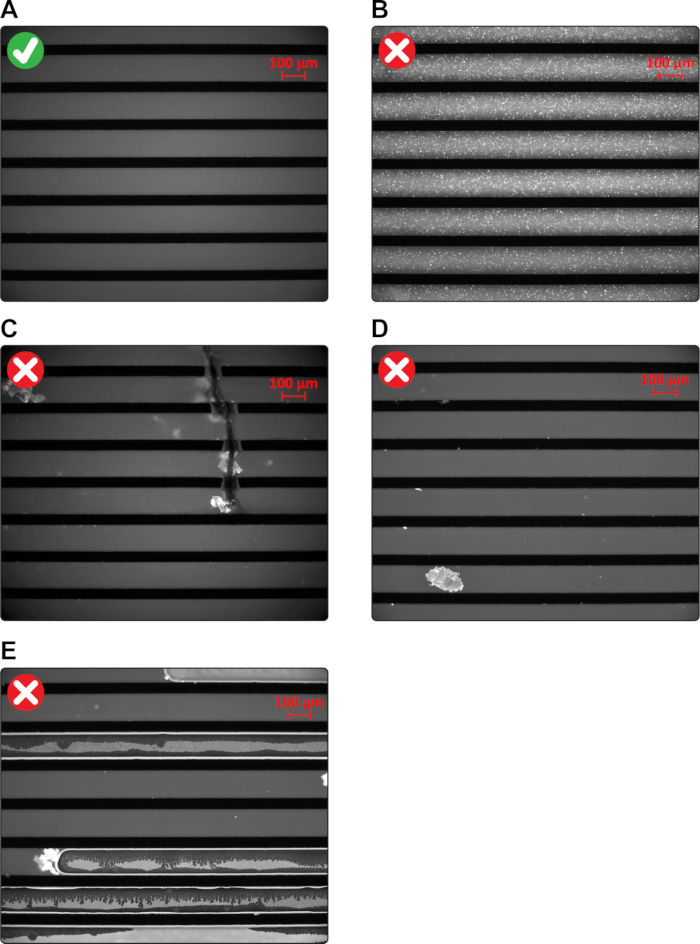
Figure 1: Assessing the integrity of SLBs and microchannels. (A) An image illustrating high-quality SLBs and microchannels. (B) Incomplete fusion. (C) Fused microchannels. (D) Dust particle trapped within a microchannel. (E) Air bubbles trapped within microchannels.
Declarações
The authors have nothing to disclose.
Materials
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine | Avanti Polar Lipids | 850457C | POPC |
| L-α-phosphatidylinositol-4-phosphate | Avanti Polar Lipids | 840045X | PI4P |
| L-α-phosphatidylinositol-4,5-bisphosphate | Avanti Polar Lipids | 840046X | PI(4,5)P2 |
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine | Avanti Polar Lipids | 850757C | POPE; Required for the synthesis of oSRB-POPE |
| Lissamine Rhodamine B Sulfonyl Chloride (mixed isomers) | ThermoFisher Scientific | L-20 | Required for the synthesis of oSRB-POPE |
| pH-Sensitive Fluorescent Lipid Probe (oSRB-POPE) | In-house | N/A | In-house Synthesis (Huang D. et al. 2013) |
| Axiovert 200M Epifluorescence Microscope | Carl Zeiss Microscopy | N/A | Microscope |
| AxioCam MRm Camera | Carl Zeiss Microscopy | N/A | Camera |
| X-Cite 120 | Excelitas Technologies | N/A | Light Source |
| Alexa 568 Filter Set | Carl Zeiss Microscopy | N/A | Ex/Em 576/603 nm |
| AxioVision LE64 v.4.9.1.0 Software | Carl Zeiss Microscopy | N/A | Image-Processing Software |
| Tips | VWR | 10034-132 | 200 uL pipette tips; Thin and smooth tip for applying the protein solution into the microfluidic channel |
| Tips | VWR | 53509-070 | 10 uL pipette tips; Thin and smooth tip for applying the vesicle solution into the microfluidic channel |
| Orion Star A321 pH meter | Thermo Scientific | STARA3210 | pH meter |
| Orion micro pH probe | Thermo Scientific | 8220BNWP | micro pH probe |
| N-(2-Hydroxyethyl)-Piperazine-N'-(2-Ethanesulfonic Acid) | VWR | VWRB30487 | HEPES, Free Acid |
| Sodium Chloride | VWR | BDH8014-2.5KGR | NaCl |
| Tubing | Allied Wire & Cable | TFT-200-24 N | Internal Diameter: 0.020-0.026 inches (0.051-0.066 cm); Wall Thickness: 0.010 inches (0.025 cm); Flexible Polytetrafluoroethylene Thin-Wall Tubing; Natural Color |
| Nitrogen Gas – Industrial | Praxair | N/A | Local Provider |
| Oxygen Gas – Industrial | Praxair | N/A | Local Provider |
| Liquid Nitrogen | Praxair | N/A | Local Provider |

