16.12:
Formation of Complex Ions
19,894 Views
•
•
A type of Lewis acid-base chemistry involves the formation of a complex ion (or a coordination complex) comprising a central atom, typically a transition metal cation, surrounded by ions or molecules called ligands. These ligands can be neutral molecules like H2O or NH3, or ions such as CN− or OH−. Often, the ligands act as Lewis bases, donating a pair of electrons to the central atom. These types of Lewis acid-base reactions are examples of a broad subdiscipline called coordination chemistry—the topic of another chapter in this text.
The equilibrium constant for the reaction of a metal ion with one or more ligands to form a coordination complex is called a formation constant (Kf) (sometimes called a stability constant). For example, the complex ion [Cu(CN)2]− is produced by the reaction
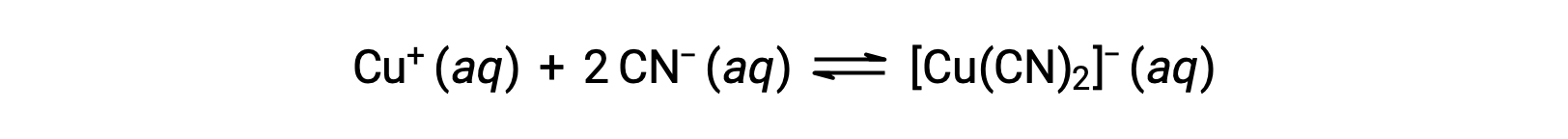
The formation constant for this reaction is
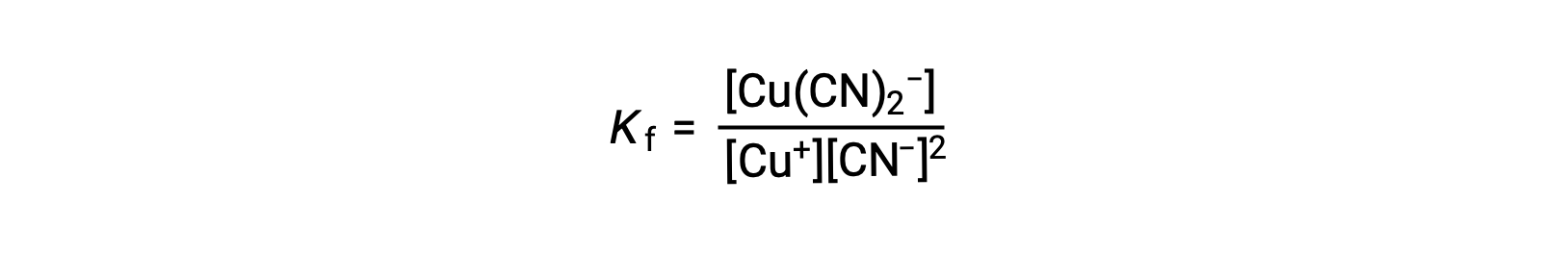
Alternatively, the reverse reaction (decomposition of the complex ion) can be considered, in which case the equilibrium constant is a dissociation constant (Kd). As per the relation between equilibrium constants for reciprocal reactions described, the dissociation constant is the mathematical inverse of the formation constant, Kd = Kf−1.
As an example of dissolution by complex ion formation, consider what happens when aqueous ammonia is added to a mixture of silver chloride and water. Silver chloride dissolves slightly in water, giving a small concentration of Ag+ ([Ag+] = 1.3 × 10−5 M):
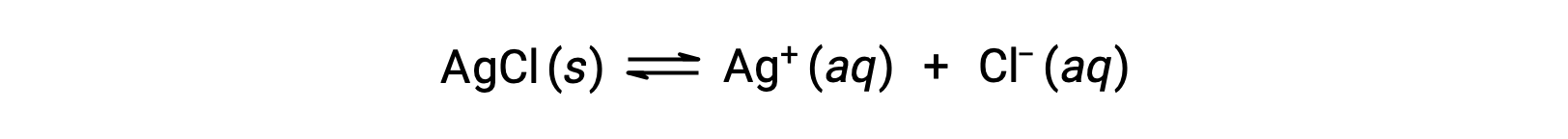
However, if NH3 is present in the water, the complex ion, [Ag(NH3)2]+, can form according to the equation:
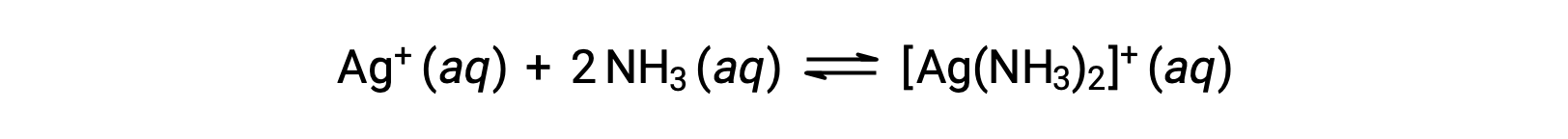
This text is adapted from Openstax, Chemistry 2e, Section 15.2: Lewis Acids and Bases.
