18.1:
Balancing Redox Equations
43,522 Views
•
•
Electrochemistry is the science involved in the interconversion of electrical and chemical reactions. Such reactions are called reduction-oxidation, or redox reactions. These important reactions are defined by changes in oxidation states for one or more reactant elements and include a subset of reactions involving the transfer of electrons between reactant species. Electrochemistry as a field has evolved to yield sufficient insights on the fundamental principles of redox chemistry and multiple technologies ranging from industrial-scale metallurgical processes to robust, rechargeable batteries for electric vehicles. As reactions involving the transfer of electrons are essential to the study of electrochemistry, a brief review of redox chemistry includes the following.
Oxidation Numbers
By definition, a redox reaction involves a change in either oxidation number or oxidation state for one or multiple reactants. The oxidation number of an element is an assessment of how the electronic environment of its atoms differs compared to atoms of the pure element. By this definition, an atom in an element carries an oxidation number of zero. For an atom, the oxidation number is equal to the atom's charge in the compound if the compound were ionic. Thus, the sum of oxidation numbers for all atoms in a molecule is equal to the molecule's charge.
Ionic Compounds
Simple ionic compounds are the simplest examples of this formalism since the elements have oxidation numbers equal to their ionic charges. Sodium chloride, NaCl, is composed of Na+ cations and Cl− anions, with oxidation numbers for sodium and chlorine being +1 and −1, respectively. Calcium fluoride, CaF2, is composed of Ca2+ cations and F− anions, with oxidation numbers for calcium and fluorine, +2, and −1.
Covalent Compounds
Covalent compounds are more challenging in the use of formalism. Water is a covalent compound consisting of two H atoms bonded to an O atom via polar covalent O−H bonds. The shared electrons making an O−H bond are more strongly attracted to the more electronegative O atom. So oxygen acquires a partial negative charge, compared to an O atom in elemental oxygen. As a result, H atoms in a water molecule exhibit a partial positive charge compared to hydrogen atoms in hydrogen gas. The sum of the partial negative and positive charges for each water molecule is zero, making the water molecule neutral.
If the polarization of shared electrons within the O−H bonds of water were complete—the result would be the complete transfer of electrons from H to O, and water would be an ionic compound composed of O2− anions and H+ cations. And so, the oxidations numbers for oxygen and hydrogen in water are −2 and +1, respectively. Applying this same logic to carbon tetrachloride, CCl4, yields oxidation numbers of +4 for carbon and −1 for chlorine. In the nitrate ion, NO3−, the oxidation number for nitrogen is +5 and that for oxygen is −2, summing to equal the −1 charge on the molecule:
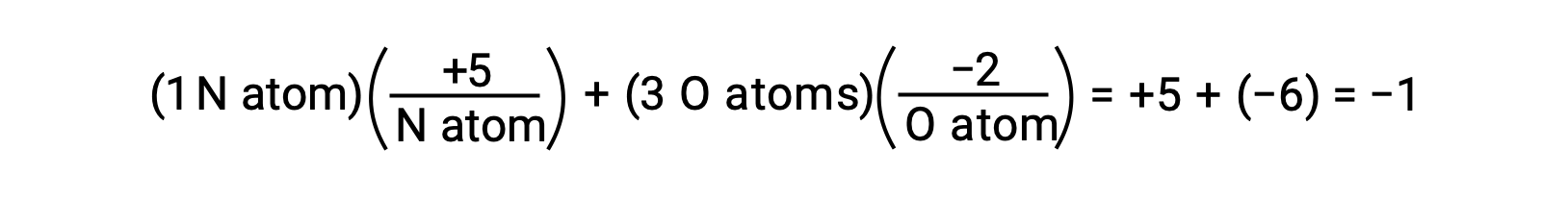
Balancing Redox Equations
The unbalanced equation shown below describes the decomposition of sodium chloride:
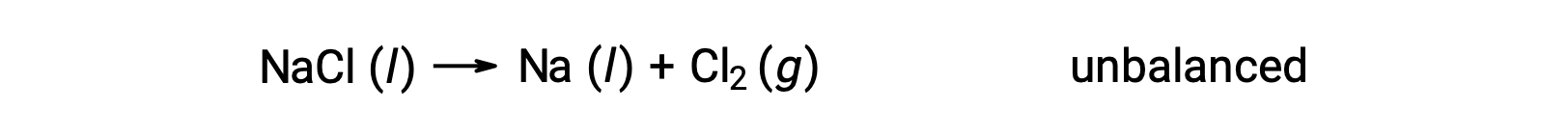
This reaction matches the criterion for a redox reaction, as the oxidation number for Na decreases from +1 to 0 (by undergoing reduction) and that for Cl increases from −1 to 0 (by undergoing oxidation). The equation case is easily balanced by adding the stoichiometric coefficient of 2 for the NaCl and Na:
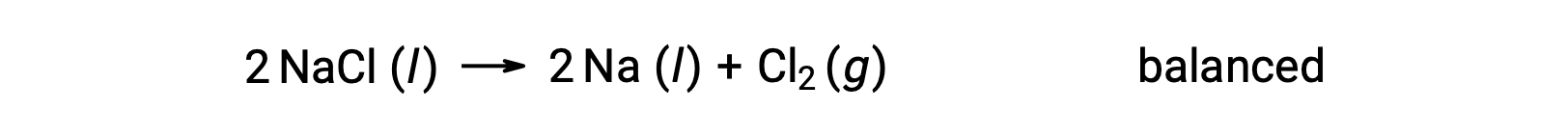
Redox reactions occurring in aqueous solutions are commonly encountered in electrochemistry, and many involve water or its ions, H+ (aq) and OH− (aq), either as reactants or products.
In these cases, equations representing redox reactions can be very challenging to balance merely by inspection, and the use of a systematic approach known as the half-reaction method is helpful. This approach involves the following steps:
- Split the equation into its component skeleton oxidation and reduction half-reactions.
- Balance each half-reaction for all elements other than O and H.
- Balance each half-reaction for O atoms by adding water molecules as needed in the equation.
- Balance each half-reaction for H atoms by adding protons as required for the equation.
- Finally, balance the charges on the elements by adding electrons as needed.
- Multiply the half-reactions by any integer needed to equalize the number of electrons lost in the oxidation half-reaction to the number of electrons gained in the reduction half-reaction.
- Add both half-reactions and simplify it further by canceling out common species on both sides of the equation.
- If the reaction occurs in an alkaline medium, add OH− ions to the equation obtained in step 7 to neutralize the protons (added in equal numbers to both sides of the equation) and simplify.
- Check the equation to ensure that the charges on the atoms are balanced.
This text is adapted from Openstax, Chemistry 2e, Chapter 17: Introduction, and Openstax, Chemistry 2e, Section17.2: Review of Redox Chemistry.
