14.1:
Dynamic Equilibrium
41,699 Views
•
•
A reversible chemical reaction represents a chemical process that proceeds in both forward (left to right) and reverse (right to left) directions. When the rates of the forward and reverse reactions are equal, the concentrations of the reactant and product species remain constant over time and the system is at equilibrium. A special double arrow is used to emphasize the reversible nature of the reaction. The relative concentrations of reactants and products in equilibrium systems vary greatly; some systems contain mostly products at equilibrium, some contain mostly reactants, and some contain appreciable amounts of both.
Dynamic Equilibrium in Chemical Reactions
Consider the reversible decomposition of colorless dinitrogen tetroxide to yield brown nitrogen dioxide, described by the equation:
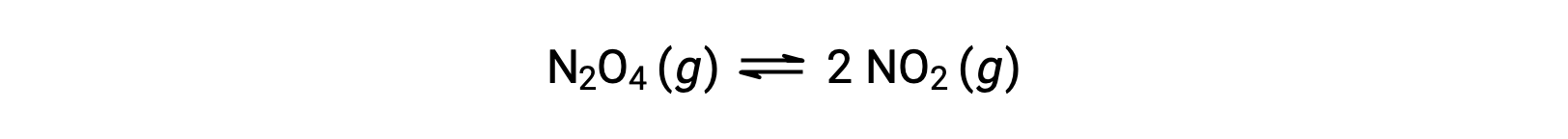
As the reaction begins (time = 0), the concentration of the N2O4 reactant is finite and that of the NO2 product is zero, so the forward reaction proceeds at a finite rate while the reverse reaction rate is zero. As time passes, N2O4 is consumed and its concentration falls, while NO2 is produced, and its concentration increases. The decreasing concentration of the reactant slows the forward reaction rate, and the increasing product concentration speeds the reverse reaction rate. This process continues until the forward and reverse reaction rates become equal, at which time the reaction has reached equilibrium. It’s important to emphasize that chemical equilibria are dynamic; a reaction at equilibrium has not “stopped,” but it is proceeding in the forward and reverse directions at the same rate. Thus, at equilibrium, the concentrations of N2O4 and NO2 no longer change because the rate of formation of NO2 is exactly equal to the rate of consumption of NO2, and the rate of formation of N2O4 is exactly equal to the rate of consumption of N2O4.
Homogeneous and Heterogeneous Equilibria
A homogeneous equilibrium is one in which all reactants and products (and any catalysts, if applicable) are present in the same phase, either aqueous or gaseous phase, as illustrated by the following examples:
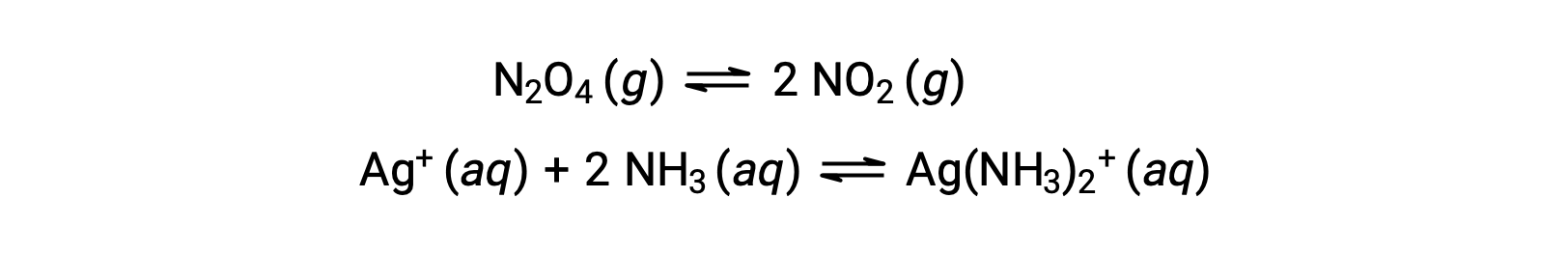
A heterogeneous equilibrium involves reactants and products in two or more different phases, as illustrated by the following example:
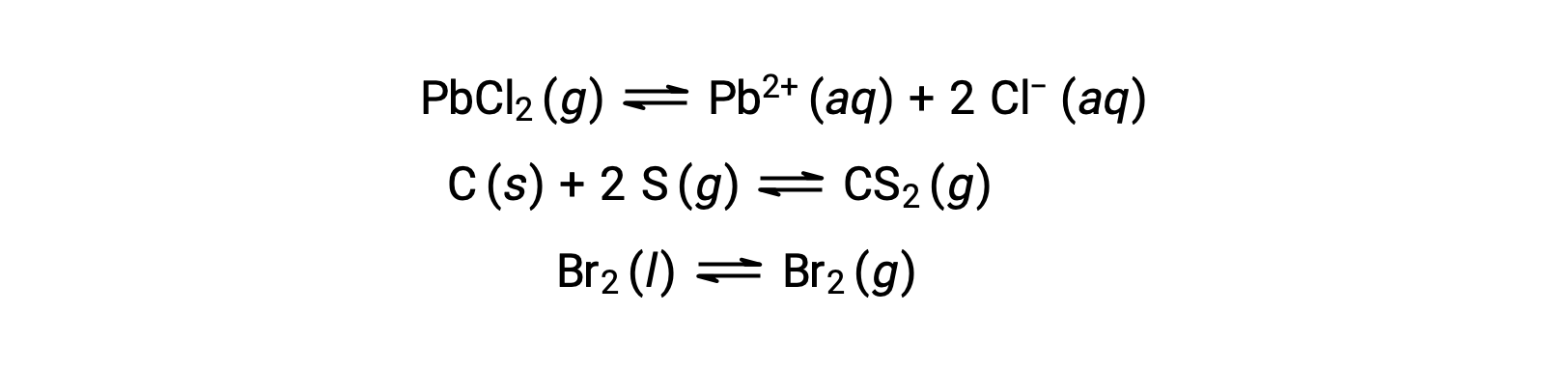
This text has been adapted from Openstax, Chemistry 2e, Section 13.1 Chemical Equilibria.
