11.15:
Molecular and Ionic Solids
14,274 Views
•
•
Crystalline solids are divided into four types: molecular, ionic, metallic, and covalent network based on the type of constituent units and their interparticle interactions.
Molecular Solids
Molecular crystalline solids, such as ice, sucrose (table sugar), and iodine, are solids that are composed of neutral molecules as their constituent units. These molecules are held together by weak intermolecular forces such as London dispersion forces, dipole-dipole interactions, or hydrogen bonds, which dictate their properties (Table 1).
The strengths of the attractive forces between the units present in different crystals vary widely, which is reflected in the melting points of such crystals.
• Small symmetrical nonpolar molecules, such as H2, N2, O2, and F2, have weak dispersion forces and form molecular solids with very low melting points (below −200 °C). Substances consisting of larger, nonpolar molecules have larger attractive forces and melt at higher temperatures.
• Molecular solids composed of polar molecules with permanent dipole moments melt at still higher temperatures. Examples include solid SO2 and table sugar. Intermolecular hydrogen bonding is mainly responsible for maintaining the three-dimensional lattice of such molecular solids, as seen in frozen water or ice.
The properties of molecular solids depend on the efficient packing of their constituent units, the molecules, in three dimensions. As the intermolecular forces are contact-dependent, higher symmetry of constituent molecules ensures close and compact packing within the crystal structure with high intermolecular attractions. This increases the melting point. The lower symmetry of the molecules prevents their efficient packing. The intermolecular forces, thus, are not as effective, and the melting point is lower.
Ionic Solids
Ionic crystalline solids, such as sodium chloride, are composed of positive and negative ions that are held together by strong electrostatic attractions.
Ionic solids have high melting points due to the strong ionic attractions. The strength of ionic interaction between the cations and the anions in an ionic solid can be approximated by the electrostatic force, given by Coulomb’s law:
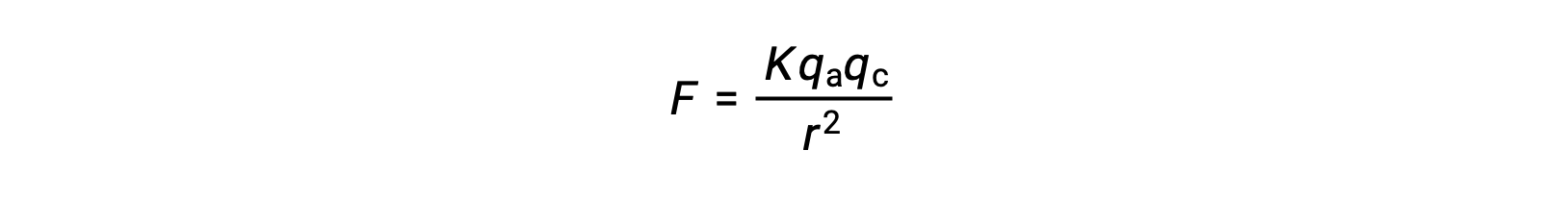
Here, K is a constant of proportionality, r is the distance between the charges, and qa and qc represent the charges on the anions and cations, respectively. Higher the charge on the cations and anions, the stronger the force of ionic attraction. Similarly, close packing of anions and cations in the crystal lattice reduces the distance between the charges, resulting in stronger forces of ionic attraction.
Ionic solids are hard, they also tend to be brittle, and they shatter rather than bend. Their brittleness is attributed to the presence of both attractive (cation-anion) and repulsive (cation–cation and anion–anion) interactions in the crystal lattice. As the ions are unable to move freely due to the strong coulombic forces, Ionic solids do not conduct electricity. However, in the molten state or when dissolved in water, the ions become free to move and conduct electricity.
Table 1. Characteristics of Molecular and Ionic Solids.
| Type of crystalline solid | Type of constituent particle | Type of attractions | Properties | Examples |
| Molecular Solids | Molecules | Intermolecular forces (IMFs): Dispersion forces, dipole-dipole forces, hydrogen bonds | variable hardness, variable brittleness, low melting points, a poor conductor of heat and electricity | Ar, H2O (ice), CO2 (dry ice), I2, C12H22O11 (sucrose) |
| Ionic Solids | Ions | Electrostatic | hard, brittle, high to very high melting points, conductor of electricity in molten and dissolved state | NaCl (table salt), MgO(Magnesium Oxide), Al2O3 (alumina) |
Part of this text has been adapted from Openstax, Chemistry 2e, Section 10.5: The Solid State of Matter.
