Separation of Mixtures via Precipitation
151,726 Views
•
•
概要
Source: Laboratory of Dr. Ana J. García-Sáez — University of Tübingen
Most samples of interest are mixtures of many different components. Sample preparation, a key step in the analytical process, removes interferences that may affect the analysis. As such, developing separation techniques is an important endeavor not just in academia, but also in industry.
One way to separate mixtures is to use their solubility properties. In this short paper, we will deal with aqueous solutions. The solubility of a compound of interest depends on (1) ionic strength of solution, (2) pH, and (3) temperature. By manipulating with these three factors, a condition in which the compound is insoluble can be used to remove the compound of interest from the rest of the sample.1
原則
A number of parameters can be used to separate a sample of interest from impurities by reducing its solubility, and removing it from a solution as a solid, as shown in Figure 1. First, changing the ionic strength of the solution can change a substances solubility. This often involves the addition of extra salt (also called salting out), or the addition of a counter-ion, which forms a less soluble species with the compound of interest.2
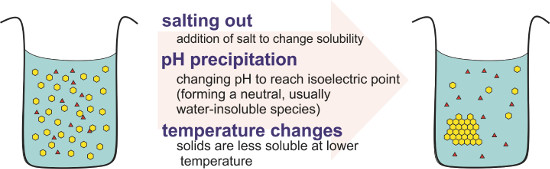
Figure 1. Solubility equilibria are affected by ionic strength, pH, and temperature. A compound of interest (yellow) is separated from impurities (red) by changing its solubility in a given solvent.
Changing the pH of a solution may change the net charge of the compound. At a certain pH, the net charge becomes zero (also called isoelectric point) and the compound becomes less soluble in water, eventually forming a solid. Temperature also affects solubility, as higher temperature increases solubility of solids.
The rate of solid formation determines relative purity (Figure 2). In general, the term precipitation refers to the formation of a solid at a rapid pace, thereby producing an amorphous sample with some impurities trapped within. This is common in salting out and pH change-induced processes. When this process is slowed down, the impurities are not trapped within the compound and a relatively pure solid is produced. This technique is employed in recrystallization. In this process, a compound is dissolved in enough solvent to be just at the saturation point at an elevated temperature. This saturated solution is then allowed to cool down slowly. As the solution cools, the solubility of the component decreases, and the compound in excess of the solubility forms a well-ordered solid (otherwise known as crystals) instead of an amorphous solid. Impurities in the solution do not get trapped as the slow process allows the removal of these impurities at the surface of the solid before they are trapped.1
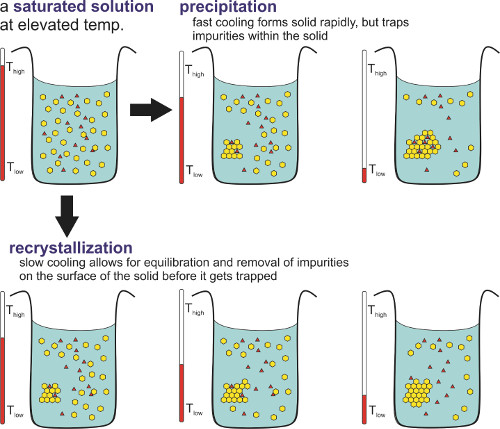
Figure 2. Difference between precipitation and recrystallization.
Once the solid has formed (whether as a crystal or as a precipitate), it should be separated from the rest of the mixture. Filtration is one way to separate them. This employs a porous material which selectively inhibits the passage of the solid material but not the solution.
Centrifugation is another way to separate the precipitate from the rest of the mixture. Centrifugation uses centripetal acceleration to separate mixtures based on their densities. Since solid is denser than the aqueous solution, the solid sediments at the bottom of the container. The solid is also called the pellet and the aqueous solution, the supernatant. The supernatant can then be decanted or extracted using a pipet or syringe. Crystals are fragile and centrifugation is often not employed to separate them from the solution.
This video will cover different methods of separating compounds through solid formation (salting out, pH changes, and recrystallization) and their subsequent removal from the aqueous solution through filtration or centrifugation.
手順
1. Precipitation of CaCO3
- Prepare 5 mL of 1 M CaCl2.
- Prepare 5 mL 1 M Na2CO3.
- In a small centrifuge tube (1.5 mL), add 750 µL of CaCl2 and 750 µL of Na2CO3.
- Wait 2 min for the reaction to occur. The solution should turn cloudy.
- Centrifuge the mixture at 10,000 × g for 5 min.
- Decant the supernatant.
- Add 1 mL of cold water to the pellet.
- Resuspend the pellet by mixing in a vortex mixer for 10 s.
- Centrifuge the mixture at 10,000 × g for 5 min.
- Decant the supernatant.
2. Precipitation of Milk Proteins
- Pour the milk in a beaker and add a stir bar.
- Warm milk gently until 40 °C in a stirring hot plate. Do not heat over 40 °C.
- Prepare a 15% (v/v) of acetic acid by mixing 7.5 mL of acetic acid and diluting in enough water to reach 50 mL.
- Immerse the electrode of a pH meter in warm milk, and monitor the pH.
- Add the acetic acid drop-wise to the milk until a pH of 4.6 is reached.
- Filtration of Milk
- Flute a piece of filter paper and place it in a funnel.
- Place the funnel in an flask, and pour the acidified milk solution into the funnel.
- As the solution is poured, the filter paper may get clogged. Using a stirring rod, agitate the solution and filter paper occasionally to unclog. If it does not improve the passage of solution, change the filter paper.
- Place a new filter paper on the bench top and transfer as much of the wet solid to the new filter paper. This should absorb more water from the solid.
- If the new filter paper gets too wet, continue to change it until there is minimal amount of wetness on the filter paper. Press it lightly to absorb more water if needed.
- Take the dried solid and re-suspend in about 70% ethanol. Filter the solid again following steps 2.6.1 to 2.6.5.
- Centrifugation of Milk (as an alternative to filtration)
- Transfer 50 mL portions of the mixture to 50-mL centrifuge tubes.
- Centrifuge at 4,500 × g for 10 min, then decant the supernatant.
- Add 50 mL 70% ethanol to the pellet.
- Using a stirring rod, resuspend the pellet in the ethanol.
- Centrifuge this suspension following step 2.7.2.
- Resuspend the pellet in buffer for further analysis such as SDS-PAGE, otherwise store it at 4°C.
3. Recrystallization of KCl
- Weigh 50 g of KCl in an Erlenmeyer flask, and add 100 mL of water
- Heat the mixture until water boils. Make sure all KCl powder is dissolved. Some impurities may not dissolve in water.
- Heat another (empty) Erlenmeyer flask along with the mixture and keep it very warm.
- Place a funnel with filter paper in the warm, empty flask.
- Pour the solution through the filter paper to remove undissolved impurities. The receiving flask is kept warm to make sure no temperature changes occur during the filtration, otherwise a crude precipitate will form. If that happens, re-heat the mixture until all the precipitate dissolves.
- Remove the flask with the solution from heat.
- Keep it in a cool place in the room and let it cool down slowly for about 30 min, or until it is no longer warm to touch.
- Once cooled down to room temperature, place the flask in an ice bath to further lower the temperature. Alternatively, one can leave the flask inside the fridge or a temperature-controlled room at 4 °C.
- Crystals can be harvested by filtering as in steps 3.4–3.5 (use a flask and funnel at room temperature).
Precipitation is a technique used to separate a mixture based on the solubility of its components. The solubility of a compound depends on the ionic strength of the solution, its pH, and temperature. Manipulation of these factors can cause a compound to become an insoluble solid, and fall out of solution. This is called precipitation.
The insoluble solid, called the precipitate, initially forms a suspension, meaning that it is well dispersed in solution. The precipitate typically agglomerates, and then is separated from the liquid by sedimentation, centrifugation, or filtration. This video will introduce several methods of separating compounds using precipitation, and demonstrate a procedure in the laboratory.
A dissolved compound can be precipitated out of solution by introducing a counter ion. For example, silver can be precipitated out of solution in the reaction between silver nitrate and sodium chloride. The nitrate ion is replaced by a counter-ion, chloride, resulting in the formation of solid silver chloride.
Increasing the salt concentration of a solution can also induce precipitation. This technique, called salting-out, is common for the isolation of proteins. At high salt concentration, water molecules are more attracted to the dissolved salt, leaving fewer to stabilize the protein. As a result, the protein molecules aggregate and form a solid.
Precipitation can also be caused by a change in pH. At high and low pH, the protein is charged and attracted to the polar solution. At a certain point, the net charge of a compound becomes zero. This is the isoelectric point, or pI. The compound is unable to interact with the polar solution, causing it to aggregate and precipitate.
Temperature also affects solubility, as higher temperature increases the solubility of solids. By decreasing temperature, dissolved compounds can re-solidify. The rate of solid formation determines relative purity.
The following experiments will demonstrate the precipitation of the protein casein from milk using pH, and further separation via filtration and centrifugation methods.
To begin this procedure, add 250 mL of milk into a beaker with a stir bar. Gently warm the milk to 40 °C on a stirring hot plate. Immerse a pH meter into the warm milk, and monitor the pH. Add acetic acid drop-wise to the milk until the pH reaches the casein isoelectric point, 4.6. Insoluble milk proteins, or curds, precipitate out of solution at the isoelectric point. Remove the curds from solution by filtration. If the filter paper gets clogged, mix with a spatula to help the solution flow through. If this does not improve the filtration, change the filter paper. Transfer the wet solid from the clogged filter paper to new filter paper. This should absorb more liquid, or whey, from the solid. Continue changing the filter paper until there is minimal wetness. Pressing lightly on the solids may help the filter paper to absorb more whey.
Re-suspend the dried milk solids in 70% ethanol to wash the phospholipids out of the curds and then repeat the filtration process. As an alternative to filtration, protein solids can also be separated using centrifugation. Centrifuge 50 mL portions of the milk mixture and decant the supernatant. Re-suspend the pellet in 50 mL of 70% ethanol to help remove the phospholipids from the curds, and repeat the centrifugation process.
The milk protein solids can then be stored or re-suspended in another solution for further analysis, such as SDS-PAGE. For more information, see our video on this technique. SDS-PAGE analysis shows that precipitation enabled the removal of most impurities from the whey. All of the casein was found in the pellet, while none was found in the supernatant.
Precipitation is a commonly used technique, which can be applied to separate a variety of mixtures or solutions.
Compounds can be precipitated from a solution using a counter ion, as in this example of the precipitation of calcium carbonate.
Calcium chloride and sodium carbonate are both soluble in the aqueous phase.
When they are mixed, the calcium and carbonate form an insoluble solid, which can be separated with centrifugation. For more information on this topic, see our education video on solubility rules.
Precipitation can be utilized in the preparation of nano-scale solids that are found in a wide range of applications in nanotechnology. In this example, nano-scale seeds were used to control the growth of nano-crystals.
The precursors were heated, reacted with trioctylphosphine selenide, and then rapidly cooled. Methanol was added to the cooled solution, in order to precipitate the solids. The crystals were then recovered by centrifugation, and the crystal structure analyzed with X-ray Diffraction.
Precipitation can also be used in the preparation of polymeric ligands for drug delivery applications. In this example, a ligand is synthesized and conjugated to platinum for use as an anticancer therapy. First, the ligand was synthesized using an amide coupling reaction. It precipitated as the reaction progressed. It was then recovered using filtration.
The solid was then purified using recrystallization, and filtered again. The ligand was then complexed with the platinum compound, dried, and then purified using fractional precipitation from water with acetone. Platinum coupling was confirmed using nuclear magnetic resonance spectroscopy. The compounds could then be studied for their efficacy and side effects as anticancer agents.
You have just watched JoVE's introduction to the separation of mixtures using precipitation. You should now understand the various methods of precipitation, and how to perform these experiments in the laboratory.
Thanks for watching!
結果
Solubility equilibria is employed in many purification processes. Calcium can be removed from water using sodium carbonate. The solubility product (Ksp) of CaCO3 is 4.8 × 10-9. Mixing 1 M of CaCl2 and 1 M of Na2CO3 produced CaCO3 precipitate. The precipitate was separated from the rest of the solution using centrifugation.
Casein (a key protein in milk) has an isoelectric point at pH 4.6 and formed insoluble curds at this pH. The curds were then separated from the rest of the solution (also called whey) using either filtration or centrifugation (Figure 3a). The curd was washed with ethanol to remove phospholipids and other water-soluble compounds that were also trapped in the curd. Centrifugation prevented loss of proteins better than filtration as there were some proteins that stuck to the filter paper. The separated components were analyzed using SDS-PAGE (Figure 3b), showing that the precipitation reaction separated most of the casein from the whey. Other milk proteins, such as globulins, precipitate together with casein. Further steps may be applied for isolating casein from the rest of the proteins.
Precipitation removes most impurities from the solid, however it can also trap some impurities within the matrix. Recrystallization is often employed to further purify a solid (Figure 4). In this experiment, the solid was mixed with a solvent in which the solid was not very soluble. The temperature of the mixture was then raised to the solvent’s boiling point and enough solid is added to saturate the hot solvent. Other insoluble impurities could then be removed via a filtration step. The hot solution was then gradually cooled to room temperature and cooled further in a refrigerator/cold room/ice bath. The slow process resulted in crystals instead of amorphous precipitate. The soluble impurities were not incorporated into the crystal lattice and the resulting crystals were relatively more pure than the crude precipitate. The crystals were then harvested using filtration and left to dry in air (or in vacuum).

Figure 3. Precipitation of milk proteins. (A) Pictures of different steps in milk protein isolation. (B) SDS-PAGE of the different samples.

Figure 4. Recrystallization of KCl.
Applications and Summary
Precipitation reactions are applied to many sample preparation processes. As mentioned before, they can be used to remove salts or specific ions depending on their solubility equilibria. They can also be used to remove proteins and other biomolecules from mixtures.
Recrystallization is often employed to further purify solids. This process removes trapped impurities within the solid. Among others, recrystallization can be used to purify salts and organic molecules.
Centrifugation and filtration techniques are applicable to most sample preparation demands to separate insoluble components from the solvent. Filtration is often used in organic chemistry to separate pure crystallized compounds from its solvent. It is also used after solid-liquid extractions in natural products chemistry or analytical chemistry. Centrifugation is often used to separate mixtures of different densities and as shown here applied to separation of milk components and precipitated salt.
In biochemistry, most processes such as protein, lipid, and DNA isolation involves precipitation reactions, centrifugation and filtration methods to purify samples. And while most of these processes have been fully standardized into commercial kits, there is still a lot of room for optimization, as different biological molecules require different conditions.
参考文献
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole, Belmont, CA (2012).
- Arakawa, T., Timasheff, S.N. Mechanism of Protein Salting In and Salting Out by Divalent Cation Salts: Balance between Hydration and Salt Binding. Biochemistry. 23, 5912-5923 (1984).
筆記録
Precipitation is a technique used to separate a mixture based on the solubility of its components. The solubility of a compound depends on the ionic strength of the solution, its pH, and temperature. Manipulation of these factors can cause a compound to become an insoluble solid, and fall out of solution. This is called precipitation.
The insoluble solid, called the precipitate, initially forms a suspension, meaning that it is well dispersed in solution. The precipitate typically agglomerates, and then is separated from the liquid by sedimentation, centrifugation, or filtration. This video will introduce several methods of separating compounds using precipitation, and demonstrate a procedure in the laboratory.
A dissolved compound can be precipitated out of solution by introducing a counter ion. For example, silver can be precipitated out of solution in the reaction between silver nitrate and sodium chloride. The nitrate ion is replaced by a counter-ion, chloride, resulting in the formation of solid silver chloride.
Increasing the salt concentration of a solution can also induce precipitation. This technique, called salting-out, is common for the isolation of proteins. At high salt concentration, water molecules are more attracted to the dissolved salt, leaving fewer to stabilize the protein. As a result, the protein molecules aggregate and form a solid.
Precipitation can also be caused by a change in pH. At high and low pH, the protein is charged and attracted to the polar solution. At a certain point, the net charge of a compound becomes zero. This is the isoelectric point, or pI. The compound is unable to interact with the polar solution, causing it to aggregate and precipitate.
Temperature also affects solubility, as higher temperature increases the solubility of solids. By decreasing temperature, dissolved compounds can re-solidify. The rate of solid formation determines relative purity.
The following experiments will demonstrate the precipitation of the protein casein from milk using pH, and further separation via filtration and centrifugation methods.
To begin this procedure, add 250 mL of milk into a beaker with a stir bar. Gently warm the milk to 40 °C on a stirring hot plate. Immerse a pH meter into the warm milk, and monitor the pH. Add acetic acid drop-wise to the milk until the pH reaches the casein isoelectric point, 4.6. Insoluble milk proteins, or curds, precipitate out of solution at the isoelectric point. Remove the curds from solution by filtration. If the filter paper gets clogged, mix with a spatula to help the solution flow through. If this does not improve the filtration, change the filter paper. Transfer the wet solid from the clogged filter paper to new filter paper. This should absorb more liquid, or whey, from the solid. Continue changing the filter paper until there is minimal wetness. Pressing lightly on the solids may help the filter paper to absorb more whey.
Re-suspend the dried milk solids in 70% ethanol to wash the phospholipids out of the curds and then repeat the filtration process. As an alternative to filtration, protein solids can also be separated using centrifugation. Centrifuge 50 mL portions of the milk mixture and decant the supernatant. Re-suspend the pellet in 50 mL of 70% ethanol to help remove the phospholipids from the curds, and repeat the centrifugation process.
The milk protein solids can then be stored or re-suspended in another solution for further analysis, such as SDS-PAGE. For more information, see our video on this technique. SDS-PAGE analysis shows that precipitation enabled the removal of most impurities from the whey. All of the casein was found in the pellet, while none was found in the supernatant.
Precipitation is a commonly used technique, which can be applied to separate a variety of mixtures or solutions.
Compounds can be precipitated from a solution using a counter ion, as in this example of the precipitation of calcium carbonate.
Calcium chloride and sodium carbonate are both soluble in the aqueous phase.
When they are mixed, the calcium and carbonate form an insoluble solid, which can be separated with centrifugation. For more information on this topic, see our education video on solubility rules.
Precipitation can be utilized in the preparation of nano-scale solids that are found in a wide range of applications in nanotechnology. In this example, nano-scale seeds were used to control the growth of nano-crystals.
The precursors were heated, reacted with trioctylphosphine selenide, and then rapidly cooled. Methanol was added to the cooled solution, in order to precipitate the solids. The crystals were then recovered by centrifugation, and the crystal structure analyzed with X-ray Diffraction.
Precipitation can also be used in the preparation of polymeric ligands for drug delivery applications. In this example, a ligand is synthesized and conjugated to platinum for use as an anticancer therapy. First, the ligand was synthesized using an amide coupling reaction. It precipitated as the reaction progressed. It was then recovered using filtration.
The solid was then purified using recrystallization, and filtered again. The ligand was then complexed with the platinum compound, dried, and then purified using fractional precipitation from water with acetone. Platinum coupling was confirmed using nuclear magnetic resonance spectroscopy. The compounds could then be studied for their efficacy and side effects as anticancer agents.
You have just watched JoVE’s introduction to the separation of mixtures using precipitation. You should now understand the various methods of precipitation, and how to perform these experiments in the laboratory.
Thanks for watching!
