Microscopy and Staining: Gram, Capsule, and Endospore Staining
351,593 Views
•
•
概要
Source: Rhiannon M. LeVeque1, Natalia Martin1, Andrew J. Van Alst1, and Victor J. DiRita1
1 Department of Microbiology and Molecular Genetics, Michigan State University, East Lansing, Michigan, United States of America
Bacteria are diverse microorganisms found nearly everywhere on Earth. Many properties help distinguish them from each other, including but not limited to Gram-staining type, shape and arrangement, production of capsule, and formation of spores. To observe these properties, one can use light microscopy; however, some bacterial characteristics (for example size, lack of coloration, and refractive properties) make it hard to distinguish bacteria solely with a light microscope (1, 2). Staining bacteria is necessary when distinguishing bacterial types with light microscopy. The two main types of light microscopes are simple and compound. The main difference between them is the number of lenses used to magnify the object. Simple microscopes (for example a magnifying glass) have only one lens to magnify an object, while compound microscopes have several lenses to enhance magnification (Figure 1). Compound microscopes have an objective lens close to the object which collects light to create an image of the object. This is then magnified by the eyepiece (ocular lens) which enlarges the image. Combining the objective lens and eyepiece allows for higher magnification than using a single lens alone. Typically, compound microscopes have multiple objective lenses of varying powers to allow for different magnification (1, 2). Here, we will discuss visualizing bacteria with Gram stains, Capsule stains, and Endospore stains.

Figure 1: A typical compound microscope. The most important parts of the microscope are labeled.
The Gram stain, developed in 1884 by the Danish bacteriologist Hans Christian Gram (1), differentiates bacteria based on the composition of the cell wall (1, 2, 3, 4). Briefly, a bacterial smear is placed on a microscope slide and then heat-fixed to adhere the cells to the slide and make them more readily accepting of stains (1). The heat-fixed sample is stained with Crystal Violet, turning the cells purple. The slide is flushed with an Iodine solution, which fixes the Crystal Violet to the cell wall, followed by a decolorizer (an alcohol) to wash away any non-fixed Crystal Violet. In the final step, a counterstain, Safranin, is added to color cells red (Figure 2). Gram-positive bacteria stain purple due to the thick peptidoglycan layer which is not easily penetrated by the decolorizer; Gram-negative bacteria, with their thinner peptidoglycan layer and higher lipid content, destain with the decolorizer and are counterstained red when Safranin is added (Figure 3). Gram staining is used to differentiate cells into two types (Gram-positive and Gram-negative) and is also useful to distinguish cell shape (spheres or cocci, rods, curved rods, and spirals) and arrangement (single cells, pairs, chains, groups, and clusters) (1, 3).

Figure 2: Schematic of the Gram Staining Protocol. The left column shows how Gram-negative bacteria react at each step of the protocol. The right column shows how Gram-positive bacteria react. Also, shown are two typical bacterial cell shapes: the bacilli (or rods) and the cocci (or spheres).

Figure 3: Gram Staining Results. A Gram stain of a mixture of Staphylococcus aureus (Gram-positive purple cocci) and Escherichia coli (Gram-negative red rods).
Some bacteria produce an extracellular viscous outer layer called a capsule (3, 5). Capsules are protective structures with various functions, including but not limited to adherence to surfaces and other bacteria, protection from desiccation, and protection from phagocytosis. Capsules are typically composed of polysaccharides containing more than 95% water, but some may contain polyalcohols and polyamines (5). Due to their mostly non-ionic composition and tendency to repel stains, simple staining methods do not work with capsule; instead, capsule staining uses a negative staining technique which stains the cells and the background, leaving the capsule as a clear halo around the cells (1, 3) (Figure 4). Capsule staining involves smearing a bacterial sample into an acidic stain on a microscope slide. Unlike Gram staining, the bacterial smear is not heat-fixed during a Capsule Stain. Heat-fixing can disrupt or dehydrate the capsule, leading to false negatives (5). Furthermore, heat-fixing can shrink cells resulting in a clearing around the cell which can be mistaken as a capsule, leading to false positives (3). The acidic stain colors the slide background; while follow up with a basic stain, Crystal Violet, colors the bacterial cells themselves, leaving the capsule unstained and appearing as a clear halo between the cells and the slide background (Figure 5). Traditionally, India ink has been used as the acidic stain because these particles cannot penetrate the capsule. Therefore, neither the capsule nor the cell is stained by India ink; instead, the background is stained. Congo Red, Nigrosin, or Eosin can be used in place of India ink. Capsule staining can help doctors diagnose bacterial infections when looking at cultures from patient samples and guide appropriate patient treatment. Common diseases caused by encapsulated bacteria include pneumonia, meningitis, and salmonellosis.

Figure 4: Schematic of the Capsule Staining Protocol. The top panel shows the slide smear prior to any stain application. The middle panel shows how the slide and bacteria look after the primary stain, Congo Red. The final panel shows how the slide and bacteria look after the counterstain, Crystal Violet.
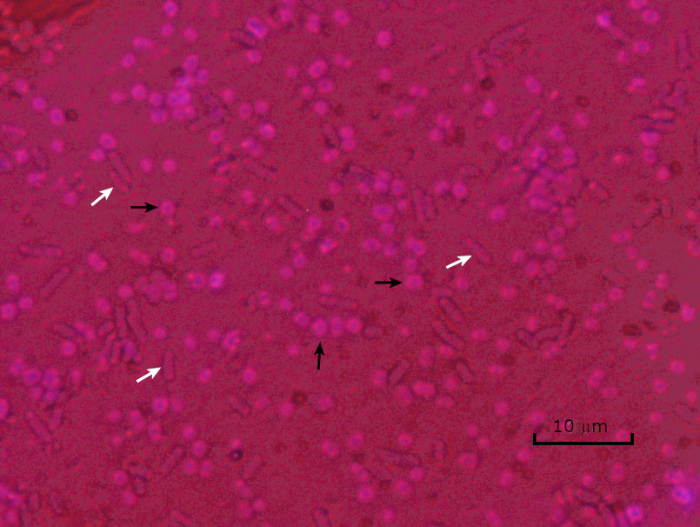
Figure 5: Capsule Staining Results. Capsule staining of encapsulated Acinetobacter baumannii (denoted with black arrows) and non-encapsulated Escherichia coli (denoted with white arrows). Notice the background is dark and A. baumannii cells are stained purple. The capsule around A. baumannii cells is evident as a halo, while E. coli has no halo.
In adverse conditions (for example nutrient limitation, extreme temperatures, or dehydration), some bacteria produce endospores, metabolically inactive structures that are resistant to physical and chemical damage (1, 2, 8, 9). Endospores allow the bacterium to survive harsh conditions by protecting the genetic material of the cells; once conditions are favorable for growth, spores germinate, and bacterial growth continues. Endospores are difficult to stain with standard staining techniques because they are impermeable to dyes typically used for staining (1, 9). The technique routinely used to stain endospores is the Schaeffer-Fulton Method (Figure 6), which uses the primary stain Malachite Green, a water soluble stain that binds relatively weakly to cellular material, and heat, to allow the stain to break through the cortex of the spore (Figure 7). These steps color the growing cells (termed vegetative cells in the context of endospore biology), as well as endospores and any free spores (those no longer within the former cell envelope). Vegetative cells are washed with water to remove Malachite Green; endospores retain the stain due to heating the Malachite Green within the spore. Finally, the vegetative cells are counterstained with Safranin to visualize (Figure 8). Staining for endospores helps differentiate bacteria into spore formers and non-spore formers, as well as determines whether spores are present in a sample which, if present, could lead to bacterial contamination upon germination.

Figure 6: Schematic of the Endospore Staining Protocol. The left column shows how spore forming bacteria react at each step of the protocol. The right column shows how non-spore forming bacteria react.

Figure 7: Diagram of Endospore Structure. Bacterial cell containing an endospore with the various spore structures labeled.
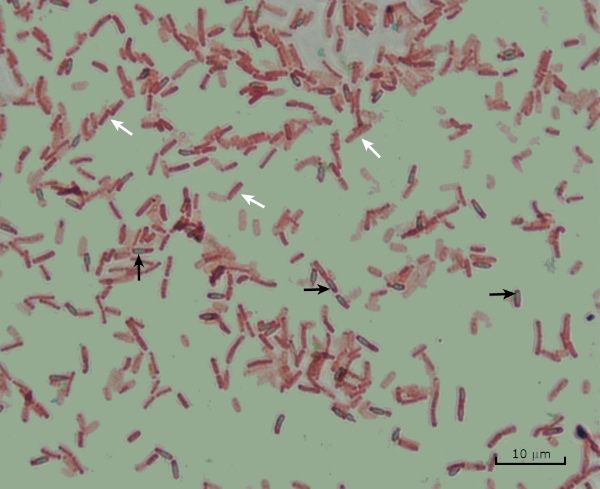
Figure 8: Endospore Staining Results. A typical staining of endospores of Bacillus subtilis. The vegetative cells (denoted with the white arrows) are stained red, while the endospores (denoted with the black arrows) are stained green.
手順
1. Gram Staining
- Set-up
- Wear gloves and a non-flammable lab coat, as dyes will stain hands and clothing.
- A Bunsen burner is used to heat-fix the bacteria. Use care when working with flame; tie back long hair.
- Commercially available Gram stain reagents will be used.
- Clean microscope slides with laboratory wipes.
- Protocol
- Pipet 10 µL phosphate-buffered saline or culture broth onto the slide.
- Smear a bacterial colony into the liquid to produce a thin, even layer.
Note: Do not use cultures older than 24 hours, as bacteria too old could have changes in their cell wall, which will affect the Gram stain results (1, 4). - Completely air-dry slide.
- Once dried, heat-fix bacteria by passing slide through the flame (bacteria side up) 4-5 times.
Note: Do not hold the slide in the flame too long or you might distort the bacterial cells (1). - Working over the sink, hold the slide level and apply Gram's Crystal Violet to completely cover the heat-fixed bacteria, allow to stand 45 seconds.
- Rinse excess Crystal Violet by holding the slide at an angle and squirting a gentle, indirect stream of water onto the slide and letting it run down over the stained bacteria. Do not squirt water directly onto the bacteria.
- Holding slide level again, apply Gram's Iodine Solution to completely cover the stained bacteria, allow to stand 45 seconds.
- Rinse excess Iodine as in step 1.2.6 above.
- While holding the slide at an angle, add a few drops of Decolorizer onto the slide, letting it trickle down over the stained bacteria just until the runoff is clear; typically, about 5 seconds. Immediately rinse with water as in step 1.2.6 above.
Note: This is a critical step in the protocol. Allowing Decolorizer to trickle too long or not long enough will result in false Gram-staining (4). - Holding the slide level again, apply Gram's Safranin to completely cover the bacteria, allow to stand 45 seconds.
- Rinse excess Safranin as in step 1.2.6 above.
- Blot, do not rub, excess water from slide using paper towels.
- Examine slide on the microscope using oil-immersion with a 100X objective.
- Results and Data Analysis
- Gram-positive bacteria will stain purple.
- Gram-negative bacteria will stain red.
- Shape (cocci, bacilli, curved rods, spirals) of bacteria will be visible.
- Arrangement of bacterial cells (single cells, paired cells, chains of cells, clusters, groupings) will be visible.
2. Capsule Staining
- Set-up
- Wear gloves and a lab coat as dyes stain hands and clothing.
- To prepare 1% Crystal Violet solution, mix 0.25 gram Crystal Violet with 25 mL distilled water until dissolved.
- To prepare 1% Congo Red solution, mix 0.25 gram Congo Red with 25 mL distilled water until dissolved.
- Clean slides with laboratory wipes.
- Protocol
- Place 10 µL Congo Red on slide.
- Using a pipet tip, smear a bacterial colony into the dye to produce a thin even layer.
- Completely air-dry slide with dye/cell mixture, 5-7 minutes.
Note: Do not heat fix as heating can dehydrate or distort the capsule. - Flood the smear with 1% Crystal Violet for 1 minute.
- Rinse excess stain by holding the slide at an angle and squirting a gentle, indirect stream of water onto the slide and letting it run down over the stained bacteria. Do not squirt water directly onto the bacteria.
- Hold the slide at a 45-degree angle until completely air-dried.
- Examine smear on the microscope under oil immersion with a 100X objective.
- Results and Data Analysis
- Bacterial cells will stain purple.
- The background of the slide will stain dark.
- Capsules will be a clear halo around cells against a dark background.
3. Endospore Staining (Schaeffer-Fulton method)
- Set-up
- Wear gloves and a non-flammable lab coat to protect hands and clothing from dyes and flame.
- A Bunsen burner is used to heat fix the bacteria. Use care when working with flame; tie back long hair.
- To prepare 0.5% Malachite Green solution, mix 0.125 grams Malachite Green with 25 mL distilled water until dissolved.
- Use commercially available Gram's Safranin reagent solution.
- Clean slides with laboratory wipes.
- Protocol
- Pipet 10 µl phosphate buffered saline (PBS) or culture broth onto slide.
- Using aseptic technique, smear a bacterial colony into the liquid to produce a thin, even layer.
Note: Endospores generally do not form in young cells; therefore, the culture is recommended to be between 18 and 36 hours old (9). - Completely air-dry slide.
- Heat fix by passing slide (bacteria side up) through flame 4-5 times.
- To help contain the dye, place a piece of lens paper (cut to fit the bacterial smear) over the heat-fixed smear.
- Saturate lens paper with Malachite Green solution.
- Place slide on top of the beaker of boiling water on a hot plate, and steam slide for 5 minutes, keeping lens paper moist by adding more dye a drop at a time as needed.
Note: Avoid overheating and drying out the dye solution. - Remove slide from beaker, remove and discard lens paper, allow slide to cool 2 minutes.
- Holding slide at an angle, rinse thoroughly by squirting a gentle, indirect stream of water onto slide, allowing it to drain down over smear.
- Holding slide level, flood smear with Safranin, allow to stand 1 minute.
- Rinse excess Safranin as in step 3.2.9 above.
- Allow to air-dry.
- Examine slide on microscope under oil-immersion with a 100X objective.
- Results and Data Analysis
- Spores will stain green.
- Vegetative cells will stain red.
- Some vegetative cells will contain spores; the cells will stain red, while the endospores will stain green.
-Bacteria are microscopic living organisms that have many distinguishing characteristics such as shape, arrangement of cells, whether or not they produce capsules, and if they form spores. These features can all be visualized by staining and aid in the identification and classification of different bacterial species.
To examine the first two characteristics of cell shape and arrangement, we can use a simple technique called Gram staining. Here, crystal violet is applied to bacteria, which have been heat-fixed onto a slide. Next, Gram's iodine solution is added to the slide, resulting in the formation of an insoluble complex between the crystal violet and the Gram's iodine solution. A decolorizer is then applied and any bacteria with a thick peptidoglycan layer will stain purple, as this layer is not easily penetrated by the decolorizer. These bacteria are referred to as Gram-positive.
Gram-negative bacteria have a thinner peptidoglycan layer and will de-stain the decolorizer, losing the purple color. However, they will stain reddish-pink when a safranin counterstain is added, which binds to a lipopolysaccharide layer on their outside. Once stained, the cells can be observed for morphology, size, and arrangement, such as in chains or clusters, which further aids in classification and identification.
Another useful technique in the microbiologist's toolkit is the capsule stain, used to visualize external capsules that surround some types of bacterial cells. Due to the capsule's non-ionic composition and tendency to repel stains, simple staining methods won't work. Instead, a negative staining technique is used, which first stains the background with an acidic colorant, such as Congo red, before the bacterial cells are stained with crystal violet. This leaves any capsule present as a clear halo around the cells.
The final major staining technique covered here can help determine if the bacteria being studied forms spores. In adverse conditions, some bacteria produce endospores, dormant, tough, non-reproductive structures whose primary function is to ensure the survival of bacteria through periods of environmental stress, like extreme temperatures or dehydration. However, not all bacterial species make endospores, and they are difficult to stain with standard techniques because they are impermeable to many dyes. The Schaeffer-Fulton method uses malachite green stain, which is applied to the bacteria fixed to a slide. The slide is then washed with water before being counterstained with Safranin. Vegetative cells will appear pinkish-red, while any endospores present will appear green. In this video, you will learn how to perform these common bacterial staining techniques and then examine the staining samples using light microscopy.
To begin the procedure, tie back long hair and put on the appropriate personal protective equipment, including a lab coat and gloves.
Then, clean a fresh microscope slide with a laboratory wipe. Next, pipette 10 microliters of 1X phosphate-buffered saline onto the first slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacterial colony in the liquid to produce a thin, even layer. Set the slide on the benchtop, and allow it to fully air dry.
Once dried, light a Bunsen burner to heat-fix the bacteria. Using tongs, pass the slide through the burner flame several times, with the bacteria side up, taking care not to hold the slide in the flame too long, which may distort the cells.
Now, working over the sink, hold the slide level and apply several drops of Gram's crystal violet to completely cover the bacterial smear and then place the slide onto the bench to stand for 45 seconds. Next, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacterial smear directly. Now, holding the slide level again, apply Gram's iodine solution to completely cover the stained bacteria and then allow it to stand for another 45 seconds. Next, carefully rinse the iodine from the slide, as shown previously. While holding the slide at an angle, add a few drops of Gram's decolorizer to the slide, allowing it to run down over the stained bacteria, just until the run-off is clear, for approximately 5 seconds. Immediately, rinse with water as shown previously. This will limit over-decolorizing the smear. Next, holding the slide level again, apply Gram's safranin counterstain to completely cover the stained bacteria. After 45 seconds, gently rinse the Safranin from the slide with water, as shown previously, and then blot dry with paper towels.
Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective lens.
To begin this staining protocol, first put on the correct personal protective equipment and then ensure that the glass slides that will be used are clean.
Next, prepare the solutions. To make 1% crystal violet solution, mix 0.25 grams of crystal violet powder with 25 milliliters of distilled water and vortex until dissolved. Then, prepare 1% Congo red solution by mixing 0.25 grams of Congo red powder with 25 milliliters of distilled water and vortex until dissolved. Now, pipette 10 microliters of the Congo red solution onto the slide. Using a clean, sterile pipette tip, select a single bacterial colony from the LB agar plate. Then, smear the bacterial colony into the dye to produce a thin, even layer. Completely air dry the bacterial slide for 5-7 minutes. Once the slide is dry, flood the smear with enough 1% crystal violet to cover the smear and let it sit for 1 minute. Now, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacteria directly. Continue holding the slide at a 45-degree angle until completely air-dried. Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective.
To perform endospore staining, first, prepare a 0.5% malachite green solution by mixing 0. 125 grams of malachite green powder with 25 milliliters of distilled water, and then vortex the solution until dissolved. Next, pipette 10 microliters of 1X PBS onto the center of the slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacteria into the liquid to produce a thin, even layer. Now, set the slide on the benchtop, and allow it to fully air dry. Once dried, light a Bunsen burner to heat-fix the bacteria. Pass the slide through the blue burner flame several times, with the bacteria side facing up. Then, once the slide has cooled, place a piece of precut lens paper over the heat-fixed smear. Next, turn on a hotplate to the highest setting, and bring a beaker of water to a boil.
Saturate the lens paper with the malachite green solution and, using tongs, place the slide on top of the beaker of boiling water to steam for 5 minutes. Keep the lens paper moist by adding more dye, one drop at a time, as needed. Next, again using tongs, pick up the slide from the beaker and remove and discard the lens paper. Allow the slide to cool for 2 minutes. Working over the sink, hold the slide at an angle, and gently squirt a stream of water onto the top of the slide. Now, hold the slide level and apply Safranin to completely cover the slide. Then, allow it to stand for 1 minute. Next, hold the slide at an angle and rinse as previously shown. Allow the slide to air dry on the benchtop. Finally, add a drop of immersion oil directly to the slide, and then examine the slide with a light microscope, with a 100X oil objective.
In the Gram staining protocol, two different colored stains can result. Dark purple staining indicates that the bacteria are Gram-positive and that they have retained the crystal violet stain. In contrast, reddish-pink staining is a characteristic of Gram-negative bacteria, which instead will be colored by the Safranin counterstain. Additionally, different shapes and arrangements of bacteria can be visualized after Gram staining. For example, it is possible to differentiate Cocci, or round bacteria, from rod-shaped Bacillus, or identify bacteria, which forms strands, compared to those which typically aggregate as clumps or occur singly.
In a capsule stained microscope image, the bacterial cells will typically be stained purple, and the background of the slide should be darkly stained. Against this dark background, the capsules of the bacteria, if present, will appear as a clear halo around the cells.
Lastly, in endospore staining, Vegetative cells will be stained red by the Safranin counterstain. If endospores are present in the sample, these will retain the malachite green stain and appear bluish-green in color.
Applications and Summary
Bacteria have distinguishing characteristics that can aid in their identification. Some of these characteristics can be observed by staining and light microscopy. Three staining techniques useful for observing these characteristics are Gram staining, Capsule staining, and Endospore staining. Each technique identifies different characteristics of bacteria and can be used to help physicians recommend treatments for patients, identify potential contaminants in samples or food products, and verify sample sterility.
参考文献
- Black, J. G. Microbiology Principles and Explorations, 4th edition. Prentice-Hall, Inc., Upper Saddle River, New Jersey. (1999)
- Madigan, M. T. and J. M. Martinko. Brock Biology of Microorganisms, 11th edition. Pearson Prentice Hall, Upper Saddle River, New Jersey. (2006).
- Leboffe, M. J., and B. E. Pierce. A Photographic Atlas for the Microbiology Laboratory, 2nd ed. Morton Publishing Company, Englewood, Colorado. (1996).
- Smith, A. C. and M. A. Hussey. Gram stain protocols. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.2886. (2005).
- Hughes, R. B. and A. C. Smith. Capsule Stain Protocols Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3041. (2007).
- Anthony, E. E. Jr. A note on capsule staining. Science 73(1890):319-320 (1931).
- Finegold, S. M., W. J. Martin, and E. G. Scott. Bailey and Scott's Diagnostic Microbiology, 5th edition. The C. V. Mosby Company, St. Louis, Missouri. (1978).
- Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. Methods for general and molecular bacteriology. ASM Press, Washington, DC. (1994).
- Hussey, M. A. and A. Zayaitz. Endospore Stain Protocol. Laboratory Protocols. American Society for Microbiology, Washington, DC. Available from: http://www.asmscience.org/content/education/protocol/protocol.3112. (2007).
筆記録
Bacteria are microscopic living organisms that have many distinguishing characteristics such as shape, arrangement of cells, whether or not they produce capsules, and if they form spores. These features can all be visualized by staining and aid in the identification and classification of different bacterial species.
To examine the first two characteristics of cell shape and arrangement, we can use a simple technique called Gram staining. Here, crystal violet is applied to bacteria, which have been heat-fixed onto a slide. Next, Gram’s iodine solution is added to the slide, resulting in the formation of an insoluble complex between the crystal violet and the Gram’s iodine solution. A decolorizer is then applied and any bacteria with a thick peptidoglycan layer will stain purple, as this layer is not easily penetrated by the decolorizer. These bacteria are referred to as Gram-positive.
Gram-negative bacteria have a thinner peptidoglycan layer and will de-stain the decolorizer, losing the purple color. However, they will stain reddish-pink when a safranin counterstain is added, which binds to a lipopolysaccharide layer on their outside. Once stained, the cells can be observed for morphology, size, and arrangement, such as in chains or clusters, which further aids in classification and identification.
Another useful technique in the microbiologist’s toolkit is the capsule stain, used to visualize external capsules that surround some types of bacterial cells. Due to the capsule’s non-ionic composition and tendency to repel stains, simple staining methods won’t work. Instead, a negative staining technique is used, which first stains the background with an acidic colorant, such as Congo red, before the bacterial cells are stained with crystal violet. This leaves any capsule present as a clear halo around the cells.
The final major staining technique covered here can help determine if the bacteria being studied forms spores. In adverse conditions, some bacteria produce endospores, dormant, tough, non-reproductive structures whose primary function is to ensure the survival of bacteria through periods of environmental stress, like extreme temperatures or dehydration. However, not all bacterial species make endospores, and they are difficult to stain with standard techniques because they are impermeable to many dyes. The Schaeffer-Fulton method uses malachite green stain, which is applied to the bacteria fixed to a slide. The slide is then washed with water before being counterstained with Safranin. Vegetative cells will appear pinkish-red, while any endospores present will appear green. In this video, you will learn how to perform these common bacterial staining techniques and then examine the staining samples using light microscopy.
To begin the procedure, tie back long hair and put on the appropriate personal protective equipment, including a lab coat and gloves.
Then, clean a fresh microscope slide with a laboratory wipe. Next, pipette 10 microliters of 1X phosphate-buffered saline onto the first slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacterial colony in the liquid to produce a thin, even layer. Set the slide on the benchtop, and allow it to fully air dry.
Once dried, light a Bunsen burner to heat-fix the bacteria. Using tongs, pass the slide through the burner flame several times, with the bacteria side up, taking care not to hold the slide in the flame too long, which may distort the cells.
Now, working over the sink, hold the slide level and apply several drops of Gram’s crystal violet to completely cover the bacterial smear and then place the slide onto the bench to stand for 45 seconds. Next, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacterial smear directly. Now, holding the slide level again, apply Gram’s iodine solution to completely cover the stained bacteria and then allow it to stand for another 45 seconds. Next, carefully rinse the iodine from the slide, as shown previously. While holding the slide at an angle, add a few drops of Gram’s decolorizer to the slide, allowing it to run down over the stained bacteria, just until the run-off is clear, for approximately 5 seconds. Immediately, rinse with water as shown previously. This will limit over-decolorizing the smear. Next, holding the slide level again, apply Gram’s safranin counterstain to completely cover the stained bacteria. After 45 seconds, gently rinse the Safranin from the slide with water, as shown previously, and then blot dry with paper towels.
Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective lens.
To begin this staining protocol, first put on the correct personal protective equipment and then ensure that the glass slides that will be used are clean.
Next, prepare the solutions. To make 1% crystal violet solution, mix 0.25 grams of crystal violet powder with 25 milliliters of distilled water and vortex until dissolved. Then, prepare 1% Congo red solution by mixing 0.25 grams of Congo red powder with 25 milliliters of distilled water and vortex until dissolved. Now, pipette 10 microliters of the Congo red solution onto the slide. Using a clean, sterile pipette tip, select a single bacterial colony from the LB agar plate. Then, smear the bacterial colony into the dye to produce a thin, even layer. Completely air dry the bacterial slide for 5-7 minutes. Once the slide is dry, flood the smear with enough 1% crystal violet to cover the smear and let it sit for 1 minute. Now, hold the slide at an angle and gently squirt a stream of water onto the top of the slide, taking care not to squirt the bacteria directly. Continue holding the slide at a 45-degree angle until completely air-dried. Finally, add a drop of immersion oil directly to the slide, and then examine the slide using a light microscope with a 100X oil objective.
To perform endospore staining, first, prepare a 0.5% malachite green solution by mixing 0. 125 grams of malachite green powder with 25 milliliters of distilled water, and then vortex the solution until dissolved. Next, pipette 10 microliters of 1X PBS onto the center of the slide. Then, use a sterile pipette tip to select a single bacterial colony from the LB agar plate. Smear the bacteria into the liquid to produce a thin, even layer. Now, set the slide on the benchtop, and allow it to fully air dry. Once dried, light a Bunsen burner to heat-fix the bacteria. Pass the slide through the blue burner flame several times, with the bacteria side facing up. Then, once the slide has cooled, place a piece of precut lens paper over the heat-fixed smear. Next, turn on a hotplate to the highest setting, and bring a beaker of water to a boil.
Saturate the lens paper with the malachite green solution and, using tongs, place the slide on top of the beaker of boiling water to steam for 5 minutes. Keep the lens paper moist by adding more dye, one drop at a time, as needed. Next, again using tongs, pick up the slide from the beaker and remove and discard the lens paper. Allow the slide to cool for 2 minutes. Working over the sink, hold the slide at an angle, and gently squirt a stream of water onto the top of the slide. Now, hold the slide level and apply Safranin to completely cover the slide. Then, allow it to stand for 1 minute. Next, hold the slide at an angle and rinse as previously shown. Allow the slide to air dry on the benchtop. Finally, add a drop of immersion oil directly to the slide, and then examine the slide with a light microscope, with a 100X oil objective.
In the Gram staining protocol, two different colored stains can result. Dark purple staining indicates that the bacteria are Gram-positive and that they have retained the crystal violet stain. In contrast, reddish-pink staining is a characteristic of Gram-negative bacteria, which instead will be colored by the Safranin counterstain. Additionally, different shapes and arrangements of bacteria can be visualized after Gram staining. For example, it is possible to differentiate Cocci, or round bacteria, from rod-shaped Bacillus, or identify bacteria, which forms strands, compared to those which typically aggregate as clumps or occur singly.
In a capsule stained microscope image, the bacterial cells will typically be stained purple, and the background of the slide should be darkly stained. Against this dark background, the capsules of the bacteria, if present, will appear as a clear halo around the cells.
Lastly, in endospore staining, Vegetative cells will be stained red by the Safranin counterstain. If endospores are present in the sample, these will retain the malachite green stain and appear bluish-green in color.
