SEM Imaging of Biological Samples
22,530 Views
•
•
概要
Source: Peiman Shahbeigi-Roodposhti and Sina Shahbazmohamadi, Biomedical Engineering Department, University of Connecticut, Storrs, Connecticut
A scanning electron microscope (SEM) is an instrument that uses an electron beam to nondestructively image and characterize conductive materials in a vacuum. As an analogy, an electron beam is to the SEM as light is to the optical microscope. The difference is that the electron microscope yields images of much higher resolution and magnification. The best optical microscopes typically have a resolution down to 200 nm, whereas SEMs usually claim a resolution of 0.5 nm. This is due to the fact that optical microscopes are limited by the diffraction of waves, a function of the wavelength, which is around 500 nm for visible light. Conversely, the SEM uses an energized electron beam, which as a wavelength of 1 nm. This characteristic makes them very dependable tools for the study of nano and microstructures. Electron microscopes also enable the study of biological samples with feature sizes too small for optical microscopy.
This demonstration provides an introduction to sample preparation and initial image acquisition of biological samples using a scanning electron microscope. In this case, a collagen-hydroxyapatite (HA) cellular scaffold will be studied. The vacuum environment of the SEM and the induced charging by the electron beam on non-conductive samples (such as organic matter) creates challenges that will be addressed in the preparation. The advantages and disadvantages of different imaging methods as they relate to resolution, depth of focus and sample type will also be discussed. The purpose of this demonstration is to give the participant more information on SEM to determine if this microscopy module is the best fit for a type of biological sample.
原則
When a high-energy electron beam (typically ranging from 5-30 keV) hits a sample, a range of signals are emitted from the sample. These interactions can be used to study topography, crystallography, electrical potential and local magnetic fields. The electrons undergo two types of scattering: elastic and inelastic. Inelastic scattering causes the emission of secondary electrons. These low energy electrons (~50 eV) are the outer shell electrons of the sample atoms that acquire just enough energy to leave the surface of the atom. The scattering of secondary electrons provides topographical information, as the energy level of the electrons leaving the sample atom is not high enough to travel through the sample. Therefore only surface level information is collected by the detector.
Elastic scattering, on the other hand, is not caused by dislodged electrons from the sample atoms. It is the principal beam of electrons after interaction with the nucleus, as seen in Figure 1. These electrons do not change their energy or speed, but they do change their direction based on their interaction with the nucleus. The detection of these electrons provides compositional information, and their varying contrast upon interaction with atoms of different atomic weights allows the user to distinguish differences in sample composition. In biological samples, this can be used to study embedded or attached nanoparticles and nanostructures with heavier atomic weights, such as gold or iron.
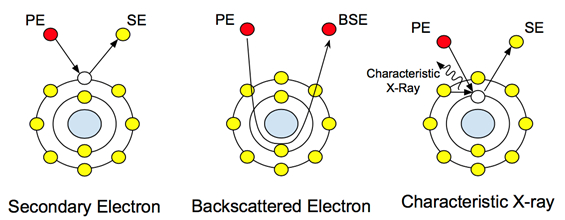
Figure 1: Atomic interactions with the principal electrons (PE) and how they create the different signals.
Sample preparation is an important procedure, especially for biological samples. In order to obtain high-resolution SEM images, electrons need to reach the sample. Then, the signals that are the result of the interaction between the electrons and the sample need to reach the detectors. This means that the instrument must work under a high vacuum to prevent electron scattering before the beam reaches the sample and the signals reach the detectors. This vacuum is highly sensitive and can pull particulate matter off of the sample, meaning it is important to make sure the sample is dry and free of particulates.
Another consideration in sample preparation is the nature of the electron beam. Because the beam is composed of highly charged particles, When a non-conductive sample is bombarded with highly charged particles from the electron beam, there is a buildup of charges on the surface that affect the deflection of the electron beam and cause a large increase in beam scatter. By coating the sample with a conductive layer before imaging, these charging artifacts in the image can be avoided.
The methods described here are applicable to most non-conductive materials. A coating is necessary to mitigate charging artifacts. The collagen-HA cellular scaffold was made by the following synthesis steps: co-precipitation of collagen with HA into the composite gel, slurry creation and freeze casting, crosslinking the scaffold and final drying. This final drying is completed over 5 days in a vacuum dryer and sufficiently dries the sample for SEM analysis without affecting the structural properties of the scaffolds. However, when imaging cells, the main concern when preparing the sample is preserving the cell structure. Chemical and resin-based fixation methods are commonly used to observe cells while preserving the structure of cells, such as glutaraldehyde fixation and epoxy and acrylic resins. Typically, glutaraldehyde is used as a fixative that creates crosslinkages in the cytoplasm of cells but also causes a drop in the pH. Therefore, buffering is needed when preparing samples with glutaraldehyde. The addition of these techniques allows the structure of the cell to most resemble its structure when it was alive [3].

Figure 2: Gold-palladium sputter coater showing the sample chamber (silver vessel on top) and vacuum (left) and current (right) gauges. In this model, a current of 2 mA is used with a chamber vacuum of 0.1 torr, which is kept constant using an argon leak valve.
手順
1. Sample Preparation
- Wear gloves and take precautions to avoid contamination when handling the sample.
- Make sure that the sample on the slide is dried and there is no contamination on the sample. This is because SEM measures surface characterization, and these defects can severely hinder the signal.
- If the sample is loaded on a standard glass slide, decrease the size of the sample by scoring the slide with a diamond tipped glass cutter in a straight line and gently push on the scored line away from the body until the glass fractures.
- Depending on the sample, choose a coating that does not have the same elemental composition (it would hinder the signal received by EDS). For this demonstration, a gold-palladium coating is used.
- Use the sputter coater as directed. Let the machine sputter the sample for around 40 s for a thin coating with adequate coverage.
- Mount the sample onto an SEM stub using conductive double-sided carbon tape. This tape should also be placed from the stage to the top of the slide that was sputtered to ground the sample if it is mounted on a non-conductive slide. A thin layer of conductive silver paint could also be used to increase the conductivity of the sample to the stage.
- Mount the stub onto the stage and tighten the screw on the side.
2. Imaging Procedure
- Load the stage into the chamber. Shut and seal the door. Then hit the "Transfer" button to open the passage from the loading chamber to the vacuum.
- Once the transfer button stops blinking and the internal door is open, the sample can be moved into the vacuum chamber by screwing in the metal rods and pushing the sample into the chamber.
- Unscrew the rod, retract and secure the rod fully into the load chamber, and press "store" to close off the load chamber from the vacuum chamber. The sample is now loaded and the rest of the process takes place from the computer workstation.
- Move the stage using the controller and by opening the stage navigation panel until it is in your field of view.
- Move the sample vertically until the working distance is 5-10 mm. When moving the stage in the z-direction, turn on the chamber camera to ensure your sample does not get to close to the electron gun.
- Turn the extra high tension (EHT) beam on. Note that you may also need to open the column if it has been off for a while.
- Select the SE2 signal from the detector options.
- Use a kV setting of about 5 kV for initial imaging, and then increase to 20-30 kV for more signal using the back-scatter mode. If the sample wasn't coated, keep the keV low to prevent too many charging artifacts in the image and to prevent sample damage.
- If there is no clear image, turn the focus, brightness, and contrast knobs until a structure is visible. This will be a reference for refinement.
- Turn the focus knob on coarse mode until an image is visible. Then switch to fine to find the best focus.
- Use stage navigation (not in the z-direction) and the magnification to find an area to save an image from.
- Decrease the scan speed and turn on line averaging to acquire a better image for saving.
- Save the image by right clicking and saving to a file location.
- Insert the BSD and move the stage back to a z-position where the sample is focused.
- Repeat steps 8-11 while looking for areas of contrast that indicate a higher atomic number.
- Remove the BSD when done.
- When ready to remove the sample, hit the button "exchange".
- Move the sample back into the load chamber and hit "store" then "vent".
Scanning electron microscopy, or SEM, is often used to image biological materials on the nano scale. Optical microscopes, which use light to image a sample, are heavily used to non-destructively image biological samples, however, their resolution and depth of field is limited, thus SEM is used in order to achieve higher resolution down to one nanometer.
In SEM, a beam of electrons is focused through a series of condenser lenses, which then hits the sample. When the beam hits the sample, electrons on the surface are scattered and measured by the detector.
In this video, we will discuss how SEM works, demonstrate how to image a biological sample in the laboratory, and finally, introduce some techniques used to image sensitive samples.
A scanning electron microscope uses a high energy electron beam, which is generated by an electron gun fitted with a filament cathode. The generated electrons are propelled toward the anode and then focused using condenser lenses before entering the objective lens. The objective lens is calibrated to focus the beam on the sample, where it is raster scanned across the surface. The interactions of the electrons with the atoms in the sample are used to study the topography, elemental composition, and crystallinity of the sample. When the incident electron beam hits the surface, it emits secondary and backscattered electrons. Secondary electrons are low energy electrons that are emitted from the sample close to the surface and provide topographical information.
Backscattered electrons, on the other hand, are reflected in the opposite direction of the incident beam. The interaction intensity increases with increasing atomic weight, enabling the user to distinguish compositional differences. Special consideration is needed to image biological samples with SEM, since SEM utilizes a high vacuum, thus biological samples, which typically have a high water content, must be dried first. This can cause collapse of the structure of sensitive samples, especially cells. Thus, cells are treated with a fixative, rinsed, and then dehydrated slowly by washing with increasing amounts of ethanol.
For rigid biological materials, such as the collagen-hydroxyapatite tissue scaffold used in this demonstration, the sample is dried over a period of several days under high vacuum.
Finally, since typical SEM imaging requires a conductive surface, biological samples are often sputter coated with a thin layer of metal prior to imaging. Now that we've discussed how SEM works and how to prepare a biological sample for imaging, let's take a look at how to prepare and image a collagen-hydroxyapatite tissue scaffold.
First, mount the biomaterial sample onto an SEM stub using conductive carbon tape and ensure that the sample is dry and has no contamination on the surface.
Then, place the mounted sample in the chamber of a sputter coater, pump down the chamber, and sputter coat the sample for around 40 seconds to achieve a thin, four- to six-nanometer thick coating of metal, in this case gold, with adequate coverage. Once coated, remove the sample and use conductive tape to connect the stub to the top of the sample, which is now coated with conductive metal.
Finally, mount the stub on the SEM stage and tighten the screw on the side. Now the sample is ready to image with SEM. First, load the stage into the SEM chamber and seal the door, then hit the transfer button to open the passage from the loading chamber to the vacuum. Once the internal door is open, screw the metal rod into the stage and push the sample into the vacuum chamber, then unscrew the metal rod and fully retract it into the load chamber, then press store to close the vacuum chamber.
Now let's image the sample using SEM.
First, move the stage using the controller and navigate the sample into the field of view, then move the sample vertically until the working distance is five to 10 millimeters. Turn the electron beam on and select the detector for secondary electrons, set the beam to five kiloelectron volts initially, then increase up to 20 to 30 kiloelectron volts as needed. If the image is not clear, turn the focus, brightness, and contrast knobs until a clear image appears.
Use the stage navigation and the X and Y directions to locate a new spot on the sample, then increase the magnification until the desired features are visible. Adjust the focus, contrast, and brightness as needed to improve the image quality. You may need to decrease the scan speed and turn on line averaging to acquire a better image, then save the image.
The SEM images reveal a highly three-dimensional and porous structure with fibrous features smaller than 25 microns. These features would be difficult to visualize using optical microscopy, as optical microscopy has a much lower depth of field.
There are many challenges associated with the imaging of biological structures with SEM, including structure collapse or damage from the high energy electron beam. Let's now take a look at how the general SEM technique is applied to these types of sensitive samples. Delicate biological structures, such as these young plant tissues, or those with a high water content, must be treated through a fixation process prior to imaging.
These floral meristems were immediately treated with a freshly prepared formalin/acetic acid fixative solution. The fixed tissue was dissected in ethanol, placed in a mesh container, and dehydrated through an ethanol series of 70%, 80%, 90%, and 100% ethanol. Finally, the plant tissues were dried using a critical point dryer, mounted, and sputter coated with a thin metal coating.
After SEM imaging, it is clear that the untreated structures were heavily damaged by the drying process and showed considerable collapse in structure, while those that were fixed maintained their native structure. Alternatively, cells and other high water content specimens can be imaged using environmental SEM, or ESEM. ESEM utilizes a high energy electron beam that is raster scanned over the sample, as with conventional SEM, however, it enables the imaging of wet or uncoated samples by maintaining a gaseous environment within the chamber.
This is done by separating the high vacuum chamber containing the electron gun from the specimen chamber using two apertures. The electron beam does incur significant losses due to scattering by gas molecules, but is typically a high enough energy for imaging. Here, cells were grown on a silicon chip, functionalized with quantum dots, and fixed using a glutaraldehyde fixation protocol. The cells were imaged in water and show the uncollapsed structure of the cell with individual quantum dots visible on the cell surface.
You've just watched JoVE's introduction to visualizing biomaterials using SEM. You should now understand how SEM works, how biological samples are prepared and imaged, as well as some applications of the technique for sensitive structures.
Thanks for watching!
結果
The SEM images in Figures 3 and 4 show that the imaged structure is highly three dimensional with microscale features. Image quality is affected by the focus and the thickness of the sputter coating.

Figure 3: The following images demonstrate how the sample focus can affect image quality. In the image on the right, the whole field of view is in focus, whereas it is out of focus on the left. Playing with parameters like the focus can provide a much better image.

Figure 4: Images of collagen-hydroxyapatite sample.
Applications and Summary
Here we demonstrated the depth of focus, field of view and maximum resolution and magnification of an electron microscope and how these properties can be used to view biological samples. This demonstration was designed to help viewers decide which microscopy module is the best for a certain application. As demonstrated, SEM has a very high depth of focus, much higher resolution and greater magnifications. However, it is not appropriate for all sample types.
This demonstration introduced the principles of electron microscopy and showed several of their applications in research labs. Electron microscopes are used for inspection, characterization and quality control. For example, they are used to visualize ICs, circuit boards, crack propagation and nano-electromechanical systems. In the field of biology these instruments play a key role as well. There are even electron microscopes especially designed to accommodate wet biological samples. These biological samples range from tissues to bone, cells and microorganisms. Using additional detectors can enable even more analysis, such as precise surface analysis.
Materials List
| Name | Company | Catalog Number | コメント |
| Equipment | |||
| Biosample | |||
| Carbon or Gold coater | |||
| Cross beam-SEM | ZEISS | ||
| Collagen-Hydroxyappetite Cellular Scaffolds | Developed by Wei Laboratory at University of Connecticut |
参考文献
- Oatley, C. W., W. C. Nixon, and R. F. W. Pease. "Scanning electron microscopy." Advances in Electronics and Electron Physics 21 (1966): 181-247.
- Goldstein, Joseph, et al. Scanning electron microscopy and X-ray microanalysis: a text for biologists, materials scientists, and geologists. Springer Science & Business Media, 2012.
- Carol Heckman, et al. Preparation of cultural cells for scanning electron microscope. Nature Protocols Network, 2007, doi:10.1038/nprot.2007.504
筆記録
Scanning electron microscopy, or SEM, is often used to image biological materials on the nano scale. Optical microscopes, which use light to image a sample, are heavily used to non-destructively image biological samples, however, their resolution and depth of field is limited, thus SEM is used in order to achieve higher resolution down to one nanometer.
In SEM, a beam of electrons is focused through a series of condenser lenses, which then hits the sample. When the beam hits the sample, electrons on the surface are scattered and measured by the detector.
In this video, we will discuss how SEM works, demonstrate how to image a biological sample in the laboratory, and finally, introduce some techniques used to image sensitive samples.
A scanning electron microscope uses a high energy electron beam, which is generated by an electron gun fitted with a filament cathode. The generated electrons are propelled toward the anode and then focused using condenser lenses before entering the objective lens. The objective lens is calibrated to focus the beam on the sample, where it is raster scanned across the surface. The interactions of the electrons with the atoms in the sample are used to study the topography, elemental composition, and crystallinity of the sample. When the incident electron beam hits the surface, it emits secondary and backscattered electrons. Secondary electrons are low energy electrons that are emitted from the sample close to the surface and provide topographical information.
Backscattered electrons, on the other hand, are reflected in the opposite direction of the incident beam. The interaction intensity increases with increasing atomic weight, enabling the user to distinguish compositional differences. Special consideration is needed to image biological samples with SEM, since SEM utilizes a high vacuum, thus biological samples, which typically have a high water content, must be dried first. This can cause collapse of the structure of sensitive samples, especially cells. Thus, cells are treated with a fixative, rinsed, and then dehydrated slowly by washing with increasing amounts of ethanol.
For rigid biological materials, such as the collagen-hydroxyapatite tissue scaffold used in this demonstration, the sample is dried over a period of several days under high vacuum.
Finally, since typical SEM imaging requires a conductive surface, biological samples are often sputter coated with a thin layer of metal prior to imaging. Now that we’ve discussed how SEM works and how to prepare a biological sample for imaging, let’s take a look at how to prepare and image a collagen-hydroxyapatite tissue scaffold.
First, mount the biomaterial sample onto an SEM stub using conductive carbon tape and ensure that the sample is dry and has no contamination on the surface.
Then, place the mounted sample in the chamber of a sputter coater, pump down the chamber, and sputter coat the sample for around 40 seconds to achieve a thin, four- to six-nanometer thick coating of metal, in this case gold, with adequate coverage. Once coated, remove the sample and use conductive tape to connect the stub to the top of the sample, which is now coated with conductive metal.
Finally, mount the stub on the SEM stage and tighten the screw on the side. Now the sample is ready to image with SEM. First, load the stage into the SEM chamber and seal the door, then hit the transfer button to open the passage from the loading chamber to the vacuum. Once the internal door is open, screw the metal rod into the stage and push the sample into the vacuum chamber, then unscrew the metal rod and fully retract it into the load chamber, then press store to close the vacuum chamber.
Now let’s image the sample using SEM.
First, move the stage using the controller and navigate the sample into the field of view, then move the sample vertically until the working distance is five to 10 millimeters. Turn the electron beam on and select the detector for secondary electrons, set the beam to five kiloelectron volts initially, then increase up to 20 to 30 kiloelectron volts as needed. If the image is not clear, turn the focus, brightness, and contrast knobs until a clear image appears.
Use the stage navigation and the X and Y directions to locate a new spot on the sample, then increase the magnification until the desired features are visible. Adjust the focus, contrast, and brightness as needed to improve the image quality. You may need to decrease the scan speed and turn on line averaging to acquire a better image, then save the image.
The SEM images reveal a highly three-dimensional and porous structure with fibrous features smaller than 25 microns. These features would be difficult to visualize using optical microscopy, as optical microscopy has a much lower depth of field.
There are many challenges associated with the imaging of biological structures with SEM, including structure collapse or damage from the high energy electron beam. Let’s now take a look at how the general SEM technique is applied to these types of sensitive samples. Delicate biological structures, such as these young plant tissues, or those with a high water content, must be treated through a fixation process prior to imaging.
These floral meristems were immediately treated with a freshly prepared formalin/acetic acid fixative solution. The fixed tissue was dissected in ethanol, placed in a mesh container, and dehydrated through an ethanol series of 70%, 80%, 90%, and 100% ethanol. Finally, the plant tissues were dried using a critical point dryer, mounted, and sputter coated with a thin metal coating.
After SEM imaging, it is clear that the untreated structures were heavily damaged by the drying process and showed considerable collapse in structure, while those that were fixed maintained their native structure. Alternatively, cells and other high water content specimens can be imaged using environmental SEM, or ESEM. ESEM utilizes a high energy electron beam that is raster scanned over the sample, as with conventional SEM, however, it enables the imaging of wet or uncoated samples by maintaining a gaseous environment within the chamber.
This is done by separating the high vacuum chamber containing the electron gun from the specimen chamber using two apertures. The electron beam does incur significant losses due to scattering by gas molecules, but is typically a high enough energy for imaging. Here, cells were grown on a silicon chip, functionalized with quantum dots, and fixed using a glutaraldehyde fixation protocol. The cells were imaged in water and show the uncollapsed structure of the cell with individual quantum dots visible on the cell surface.
You’ve just watched JoVE’s introduction to visualizing biomaterials using SEM. You should now understand how SEM works, how biological samples are prepared and imaged, as well as some applications of the technique for sensitive structures.
Thanks for watching!
