Alleviation of Diabetic Tendon Injury via Activation of Tendon Fibroblasts Autophagy under Berberine Treatment
Abstract
Berberine (BBR) is an isoquinoline alkaloid isolated from Coptis chinensis and possesses valuable pharmacological activities, including anti-inflammatory, anti-tumor, and alleviating several complications of type 2 diabetes mellitus (T2DM). However, the role of BBR in regulating diabetic tendon injury remains poorly understood. In this study, a rat model of T2DM was constructed, and cell apoptosis and autophagy were assessed in tendon tissues after BBR treatment through TdT-Mediated dUTP nick-end labeling (TUNEL) assay and immunohistochemical analysis. Tendon fibroblasts were obtained from the rat Achilles tendon, and the role of BBR in regulating cell apoptosis, the production of inflammatory cytokines, and autophagy activation were assessed using flow cytometry, quantitative real-time PCR (qRT-PCR), and western blot analysis. We demonstrated that BBR treatment significantly increased autophagy activation and decreased cell apoptosis in tendon tissues of T2DM rats. In tendon fibroblasts, BBR repressed High glucose (HG)-induced cell apoptosis and production of proinflammatory cytokines. HG treatment resulted in a decrease of autophagy activation in tendon fibroblasts, whereas BBR restored autophagy activation. More important, pharmacological inhibition of autophagy by 3-MA weakened the protective effects of BBR against HG-induced tendon fibroblasts injury. Taken together, the current results demonstrate that BBR helps relieve diabetic tendon injury by activating autophagy of tendon fibroblasts.
Introduction
Diabetes (diabetes mellitus, DM) is a systemic metabolic disorder characterized by hyperglycaemia1. At present, diabetes has become one of the main diseases threatening human health and life expectancy2. More than 90% of cases are type 2 diabetes, a metabolic disease characterized by chronic inflammation3, insulin resistance4, and damage to islet β cells5, and the prevalence is increasing each year worldwide.
Type 2 diabetes brings a series of serious complications, which have serious effects on the cardiovascular system6, the eye7, the kidney7, and nerves8, putting diabetic patients at risk for multiple disabilities and even life-threatening health risks. There have been few studies on the musculoskeletal system, especially on the pathological changes in diabetic tendons. In recent years, the incidence of chronic tendinopathy has increased significantly. Tendons can dynamically regulate their capacity to store and deliver energy9,10. In tendon tissues, tendon fibroblasts play an important role in modulating tendon adaption and tendon repair after injury9,11. At present, the function of tendon fibroblasts on tendon injury remains unclear.
As an isoquinoline alkaloid, BBR exerts pharmacological effects in a variety of physiological processes, including lowering blood glucose, lowering lipids, lowering cholesterol, anti-inflammatory effects, antibacterial effects, removing reactive oxygen species, and antagonizing nervous system dysfunction12,13. BBR can increase the uptake and utilization of glucose in adipose tissue and skeletal muscle cells, upregulate the expression of the insulin receptor in liver and skeletal muscle cells, increase the expression of LDL receptor in the liver, and reduce the levels of cholesterol and sugar in plasma14. Although BBR possesses valuable pharmacological activities in alleviating several complications of DM15,16,17, the role of BBR in regulating diabetic tendon injury remains poorly understood.
Autophagy must occur at the baseline level in most tissues to withdraw damaged organelles and provide metabolites to maintain metabolic homeostasis18,19. Autophagy acts as a crucial role in β-cell health, and impaired autophagy is correlated with β-cell dysfunction and diabetes progression20,21. Emerging studies have demonstrated that BBR-induced activation of autophagy contributes to improving diabetic nephropathy22. Based on the above findings, we explored whether BBR is helpful in alleviating diabetic tendon injury through regulating autophagy. The current results demonstrated that BBR reduced HG-induced tendon fibroblasts injury and the inflammatory response. HG decreased autophagy activation of tendon fibroblasts, whereas BBR treatment restored autophagy activation and resulted in a subsequent increase of tendon fibroblasts viability and reduction of proinflammatory cytokines.
Protocol
This study was approved by the Research Ethics Committee at the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine. All animal experiments were approved by the Ethics Committee of the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (IACUC number: YYLAC-2019-1). Male Wistar rats (200-240 g, 8 weeks old) were purchased from Shanghai SLAC Laboratory Animal Center.
1. Rat model of T2DM
- Maintain male Wistar rats (200-240 g, 8 weeks old) in a climate-controlled environment with a 12 h light/dark cycle (20 ± 2 °C and 50%-60% relative humidity). Provide food and water ad libitum during the experimental period.
- Make efforts to minimize animal suffering, including gentle handling, daily cage cleaning, and monitoring.
- Randomly assign the rats to 3 groups: the control group (n = 5), DM model group (n = 5), and diabetic model treated with BBR group (n = 5).
- Establish the rat DM model according to a previous description23.
- Administer the rats with a single intravenous injection of streptozotocin (STZ) dissolved in freshly prepared sodium citrate buffer (w/v: 2%) at a dose of 30 mg/kg. Inject the control group intraperitoneally with an equal volume of citrate sodium citrate buffer without STZ.
- Assess the blood glucose using a blood gas analyzer. Use the rats with the indicated blood glucose level (≥16.7 mmol/L, continuously for 10 days) for the T2DM model.
- After 1 week, randomly divide the T2DM rats into two groups (n = 5 of each group): untreated rats or rats administered 200 mg/kg/day of BBR by gavage for 4 weeks.
2. Primary tendon fibroblasts
- Sacrifice the rats under anesthesia through intraperitoneal injection of barbiturate (40 mg/kg) and obtain the Achilles tendon as previously reported24.
- Isolate tendon fibroblasts from tendon tissues25.
- Shredthe tendon tissues manually and place them in DMEM containing 0.2% type II collagenase. Agitate vigorously for 3 h at 37 °C.
- Remove the medium by centrifugation and add DMEM containing 10% FBS and 1% penicillin/streptomycin to the digested tissue.
- Filter the tendon tissues by a 100 µm strainer, pour the filtered solution into 6-well plates, and maintain the tendon fibroblasts in a humidified incubator at 37 °C with 5% CO2.
3. Cell viability assay
NOTE: Cell counting kit-8 (CCK-8) assay was used to measure cell viability according to the manufacturer's instructions.
- After trypsinization, plate the tendon fibroblasts in 96-well plates (4 x 103 cells/well) and then treat them with different doses of glucose (0, 5, 10, 20, 30, and 50 mM) in the presence or absence of BBR (0, 5, 10, 20, 40, and 80 µM) for 48 h.
NOTE: Glucose and BBR were dissolved in DMEM. - Add CCK-8 solution (10 µL) to each well, and incubate the cells for another 2 h at 37°C.
- Subsequently, measure the absorbance of each well with a microplate reader at a wavelength of 450 nm.
4. Cell apoptosis analysis
NOTE: A propidium iodide (PI) and annexin V-FITC flow cytometry assay was used to analyze the apoptosis rate of tendon fibroblasts.
- Seed the tendon fibroblasts (5 x 105 cells) in 6-well plates in DMEM for 24 h.
- Aspirate and discard DMEM from each well. Treat the cells with fresh DMEM containing HG (30 mM) in the presence or absence of BBR (20 µM) for 24 h.
- Detach the cells with 0.25% trypsin in 1x PBS, harvest the cells with PBS, and centrifuge at 2000 x g for 5 min.
- Resuspend the cells in binding buffer (Table of Materials) and stain with 10 µL of FITC-conjugated annexin V and 5 µL of PI in the dark for 15 min at room temperature (RT).
- Then, analyze the cells by a flow cytometer.
5. Quantitative real-time polymerase chain reaction (qRT-PCR)
- Harvest the tendon fibroblasts and homogenize using an RNA extraction kit (Table of Materials) according to the manufacturer's recommendations.
- Perform reverse transcriptional PCR with Moloney's murine leukemia virus reverse transcriptase and Oligo (dT) primers (Table of Materials).
- Carry out qRT-PCR using SYBR green qPCR Mix on real-time PCR System. The cycle conditions are denaturation at 95 °C for 10 min and 45 cycles at 95 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s.
NOTE: For details of the primers for IL-1β, IL-6 and IL-10 refer to the Table of Materials. β-actin was used as the reference gene. - Calculate the relative expression level using the 2-ΔΔCT formula, as previously described26 .
6. Western blot analysis
- After treatment with HG (30 mM) in the presence or absence of BBR (20 µM) for 24 h, lyse the tendon fibroblasts with RIPA buffer (Table of Materials), and quantify the total protein concentration using the bicinchoninic acid assay.
- Separate equivalent amounts of proteins (50 µg) from each sample by 10% SDS-PAGE at RT and then transfer to polyvinylidene fluoride (PVDF) membranes at 4 °C for 2 h.
- Block the membranes in 5% non-fat dried milk in TBST and then incubate overnight at 4 °C with the following primary antibodies: anti-LC3B (1:1500), anti-p62 (1:2000), and anti-β-actin (1:3500) antibody.
- After being washed with TBST, incubate the membranes with goat anti-rabbit H&L HRP-conjugated secondary antibodies (1:5000) at RT for 1 h.
- Use an ECL chemiluminescence kit to visualize the specific blots and quantify the autoradiograms by densitometry.
7. Immunohistochemical analysis (IHC)
- Cut the paraffin-embedded foot tendon tissues into 6 µm-thick sections.
- After fixing the sections in 4% formalin, incubate the sections with anti-LC3 antibody (1:200) overnight at 4 °C.
- After washing three times using 10 mM PBS (pH7.4 with Tween 20), incubate all sections with goat anti-rabbit HRP-conjugated secondary antibody (1:1000) for 1 h at 37 °C and stain with DAB and hematoxylin for 60 min at RT.
- Image the stained slides at 20x magnification using an inverted microscope.
8. TUNEL assay
NOTE: Cell apoptosis in tendon tissue was analyzed using a TUNEL assay kit according to the manufacturer's instructions.
- Cut the paraffin-embedded foot tendon tissues into 6 µm-thick sections.
- De-paraffinize the sections in xylene and rehydrate them in a graded series of ethanol.
- After immersing with 3% hydrogen peroxide at RT, incubate the sections with TUNEL reaction mixture for 1 h at 37 °C.
- Counterstain the nuclei using DAPI. Observe the stained cells under a fluorescence microscope (20x) and determine the percentage of TUNEL-positive cells.
9. Statistical analysis
- Use appropriate software applications to perform statistical analysis
NOTE: Here, the data are presented as the mean ± standard deviation (SD) of three independent experiments. Statistical analyses were performed with SPSS 23.0. Student's t-test was performed to compare differences between groups, and one-way analysis of variance (ANOVA) was performed for multiple group analyses. The difference was statistically significant when p < 0.05.
Representative Results
To evaluate the pharmacological role of BBR in relieving diabetic tendon injury, cell apoptosis and autophagy activation in foot tendon tissues of DM rats were assessed in the presence or absence of BBR. Figure 1A showed that the protein level of LC3 (an autophagy marker) was decreased in tendons tissues of DM rats compared with control rats, whereas BBR treatment significantly restored autophagy activation. In addition, cell apoptosis was elevated in tendons tissues of DM rats compared with normal tissues, whereas BBR significantly decreased cell apoptosis (Figure 1B). These results demonstrate that BBR increases autophagy activation and decreases cell apoptosis in tendon tissues of DM rats.
Then the role of BBR in regulating tendon fibroblasts viability and apoptosis was assessed in vitro. Figure 2A showed that tendon fibroblast viability was decreased in a dose-dependent manner and that 30 mM of HG was suitable to induce tendon fibroblast damage. The protective role of BBR against tendon fibroblast in the presence of HG was next investigated. As shown in Figure 2B, pre-treatment of tendon fibroblasts with BBR contributed to an increase in cell viability in the presence of HG (30 mM) in a dose-dependent manner. BBR (20 µM) was used to treat tendon fibroblasts in the follow-up study. Furthermore, tendon fibroblast apoptosis was measured in the presence of HG or/and BBR using flow cytometry. Figure 2C,D revealed that the proportion of apoptotic cells was significantly increased after HG treatment, whereas BBR decreased HG-induced tendon fibroblast apoptosis.
Chronic inflammation is a characteristic of type 2 diabetes that is linked to diabetic complications and contributes to insulin resistance. Therefore, the inflammatory factors IL-1β, IL-6, and IL-10 in the HG-treated tendon fibroblasts were measured to determine the effect of BBR on the inflammatory response. The results from qRT-PCR showed that the proinflammatory cytokines IL-1β and IL-6 were significantly increased. In contrast, anti-inflammatory cytokine IL-10 was decreased after HG treatment (Figure 3A–C), indicating that HG triggered a proinflammatory response in tendon fibroblasts. More important, BBR treatment repressed HG-induced proinflammatory response in tendon fibroblasts (Figure 3A–C).
To explore the role of BBR in regulating autophagy activation of tendon fibroblasts, tendon fibroblasts were treated with HG in the presence or absence of BBR, and then autophagy activation was assayed. As shown in Figure 4A, HG treatment decreased autophagy activation in tendon fibroblasts as evidenced by decreased LC3 green puncta, whereas BBR obviously restored the activation of autophagy in HG-treated tendon fibroblasts. Furthermore, the autophagy marker LC3-II and autophagy substrate p62 were assessed using western blot analysis. Figure 4B,C showed that HG decreased LC3  protein expression and enhanced p62 protein expression in tendon fibroblasts, indicating that HG inactivated autophagy of tendon fibroblasts. As expected, BBR restored autophagy activation in HG-treated tendon fibroblasts (Figure 4B,C).
protein expression and enhanced p62 protein expression in tendon fibroblasts, indicating that HG inactivated autophagy of tendon fibroblasts. As expected, BBR restored autophagy activation in HG-treated tendon fibroblasts (Figure 4B,C).
Finally, whether autophagy-mediated the role of BBR in alleviating HG-induced injury of tendon fibroblasts was investigated. To this end, a specific inhibitor of autophagy, 3-MA, was used to inactivate autophagy in the presence of BBR, and then cell viability and proinflammatory cytokines were assessed. As shown in Figure 5A,B, pharmacological inhibition of autophagy increased tendon fibroblast apoptosis compared with BBR treatment alone. Similarly, 3-MA treatment resulted in a significantly decreased tendon fibroblast viability compared with BBR treatment alone (Figure 5C). 3-MA treatment also increased the level of proinflammatory cytokines compared with BBR treatment alone (Figure 5D). Taken together, these data demonstrate that BBR relieves diabetic tendon injury by activating autophagy of tendon fibroblasts.
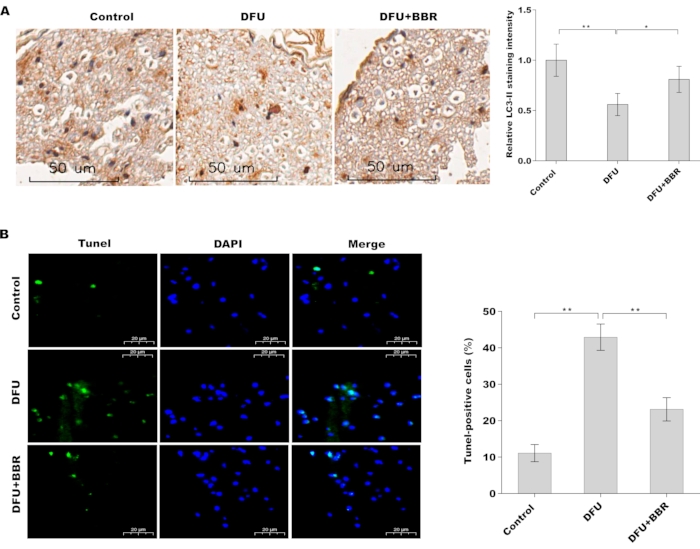
Figure 1: BBR increased autophagy activation and decreased cell apoptosis in tendon tissues of DM rats. (A) Immunohistochemical analysis of LC3 protein expression in foot tendon tissues of control rats (n = 5), DM rats (n = 5), and BBR-treated DM rats (n = 5). Scan bar = 50 µm. Magnification 20x. (B) Cell apoptosis was analyzed using TUNEL staining in foot tendon tissues of control rats (n = 5), DM rats (n = 5), and BBR-treated DM rats (n = 5). Scan bar = 20 µm. Magnification 20x. Please click here to view a larger version of this figure.
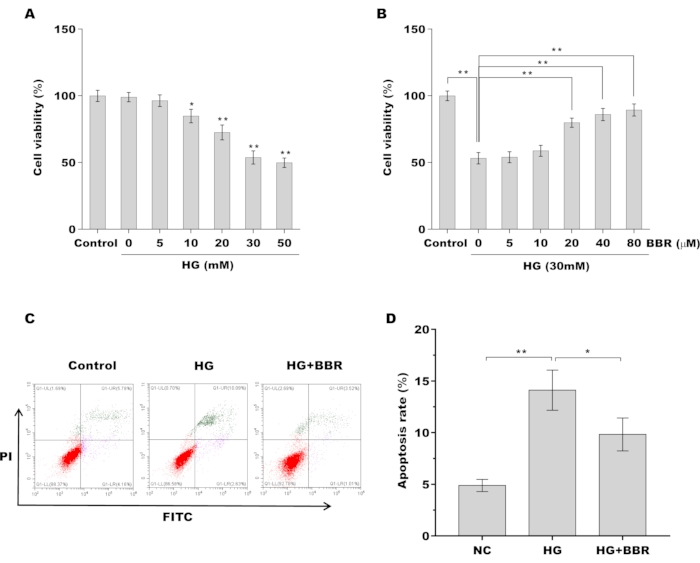
Figure 2: BBR decreased HG-induced apoptosis of tendon fibroblasts (A) Tendon fibroblast viability was assessed using CCK-8 assay after treatment with different doses of HG (0, 5, 10, 20, 30, 50 mM). (B) Tendon fibroblast viability was assessed using CCK-8 assay after treatment with 30 mM of HG in the presence of BBR (0, 5, 10, 20, 40, and 80 µM). (C,D) Cell apoptosis of tendon fibroblasts was assessed using flow cytometry after treatment with HG (30 mM) in the presence or absence of BBR (20 µM). * p < 0.05, ** p < 0.01. Please click here to view a larger version of this figure.
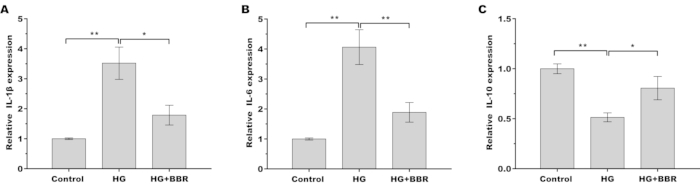
Figure 3: BBR alleviated HG-induced inflammatory response in tendon fibroblasts. (A–C) The mRNA levels of the inflammatory factors (A) IL-1β, (B) IL-6, and (C) IL-10 in tendon fibroblasts were measured by qRT-PCR after treatment with HG (30 mM) in the presence or absence of BBR (20 µM). * p < 0.05, ** p < 0.01. Please click here to view a larger version of this figure.
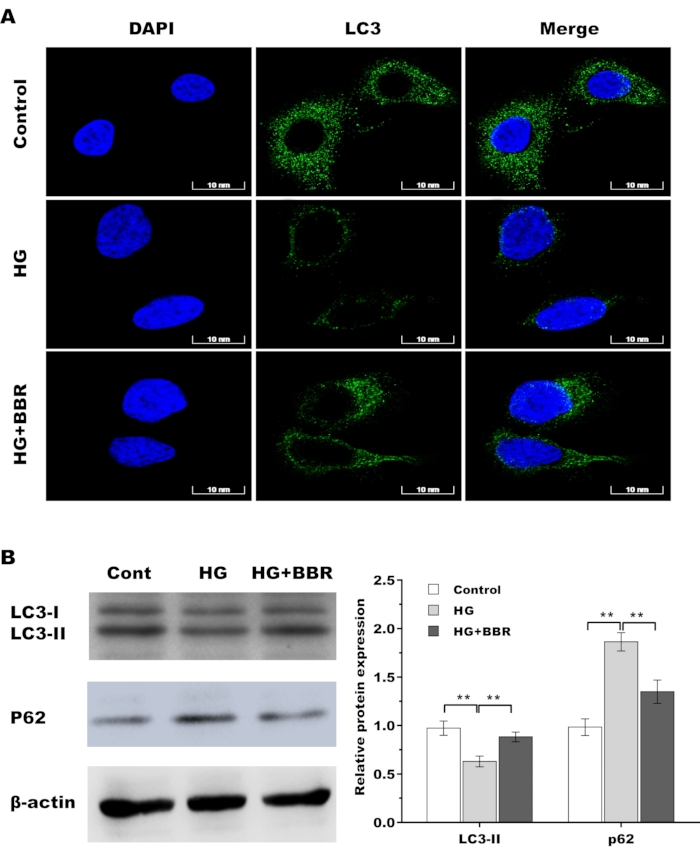
Figure 4: BBR restored autophagy activation in HG-treated tendon fibroblasts. (A) The LC3 level was assessed by immunofluorescence analysis in tendon fibroblasts after treatment with HG (30 mM) in the presence or absence of BBR (20 µM). Scan bar = 10 nm. Magnification 60x. (B) Western blot analysis for the protein levels of LC3 and P62 in tendon fibroblasts after treatment with HG (30 mM) in the presence or absence of BBR (20 µM). * p < 0.05, ** p < 0.01. Please click here to view a larger version of this figure.
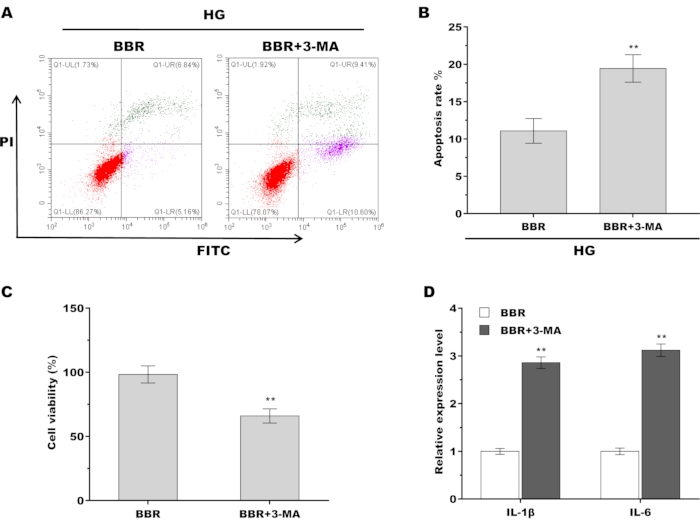
Figure 5: BBR alleviated HG-induced injury of tendon fibroblasts by activating autophagy. (A,B) Cell apoptosis of tendon fibroblasts was assessed using flow cytometry after treatment with BBR (20 µM) alone or BBR plus 3-MA (5 Mm) in the presence of HG (30 mM). (C) Tendon fibroblast viability was assessed using CCK-8 assay after treatment with BBR (20 µM) alone or BBR plus 3-MA (5 Mm) in the presence of HG (30 mM). (D) qPCR analysis of the IL-1β and IL-6 mRNA level in tendon fibroblasts after treatment with BBR (20 µM) alone or BBR plus 3-MA (5 Mm) in the presence of HG (30 mM). ** p < 0.01. Please click here to view a larger version of this figure.
Discussion
Tendon injury is a common complication in patients with DM27. Tendon fibroblasts play an important role in the wound healing process28,29. The current study verified that i) BBR increased autophagy activation and decreased cell apoptosis in tendon tissues of DM rats, ii) BBR decreased HG-induced apoptosis of tendon fibroblasts, iii) BBR alleviated HG-induced inflammatory response in tendon fibroblasts, iv) BBR restored autophagy activation in HG-treated tendon fibroblasts, v) BBR alleviated HG-induced injury of tendon fibroblasts by activating autophagy. The current results revealed the important role of BBR in regulating tendon fibroblasts autophagy in tendon injury and might provide a novel opportunity for the treatment of diabetic tendon injury.
In the study, a rat model of T2DM was used to investigate the protective role of BBR against tendon injury. The T2DM model was successfully established through a single intravenous injection of STZ at a dose of 30 mg/kg as previously described23,30. This is a critical step in evaluating the effect of BBR on alleviating tendon injury. In order to verify the model, blood glucose was assayed, and rats with the indicated blood glucose level (≥16.7 mmol/L, continuously for 10 days) were used for subsequent study. There was a small modification in constructing T2DM model. Here 30 mg/kg of STZ, instead of 40 mg/kg, were applied to treat rats. Although the rat model of T2DM has been widely used in the experimental study, few studies have applied the model in tendon injury.
Previous studies have shown that chronic low-grade inflammation (CLGI) is related to the development of T2DM and contributes to insulin resistance. Notably, CLGI is an essential factor that contributes to a variety of diabetic complications, such as diabetic foot disease, cardiovascular damage, kidney disease, retinopathy, and chronic wounds. A previous study demonstrated that tendon homeostasis was modified by HG, which repressed the expression of downstream genes of the AMPK/Egr1 pathway in tendons31. Autophagy is an effective regulator of innate immune responses, and that autophagy could repress the activation of inflammasomes and production of proinflammatory cytokines32. Hudgens et al. demonstrated that platelet-rich plasma triggers a proinflammatory response in tendon fibroblasts, which results in activation of oxidative stress pathways33. The current study further demonstrated that HG treatment induces a proinflammatory response in tendon fibroblasts, whereas BBR decreases HG-induced secretion of proinflammatory cytokines. More important, BBR represses proinflammatory response in tendon fibroblasts by activating autophagy. Moreover, BBR also represses HG-induced apoptosis of tendon fibroblasts by activating autophagy.
BBR has been widely used in the clinical treatment of metabolic diseases to improve insulin resistance and reduce glucose and lipids and as adjuvant therapy for hypertension, hyperlipidemia, diabetes, obesity, fatty liver, and other conditions. For example, BBR could decrease retinal Müller cell apoptosis in the presence of HG34. In diabetic heart disease, BBR administration can improve cardiac fibrosis and dysfunction by restraining IGF-1R, collagen I, and α-SMA levels in the heart35. In diabetic peripheral neuropathy, BBR reduces HG-induced neuroinflammation and neuronal injury by suppressing ROS generation and proinflammatory factor production36,37. BBR also regulates the inflammatory response and inhibits glomerular cell apoptosis, thus delaying the development of diabetic nephropathy38. In the present study, the data demonstrated that BBR attenuated HG-induced tendon injury, and the protective effect of BBR was mediated by activating the autophagy of tendon fibroblasts. Although BBR possesses good therapeutic roles in many disorders, its clinical application is limited by poor absorption through oral administration or severe side effects (blood pressure reduction and respiration ceased) through intravenous administration39,40.
開示
The authors have nothing to disclose.
Acknowledgements
This study was funded by Shanghai three-year Action Plan Project for Further Accelerating the Development of Traditional Chinese Medicine [ZY (2018-2020)-CCCX-4005]
Materials
| 1% penicillin/streptomycin | Sigma-Aldrich, St. Louis, MO, USA | 516104-M | |
| anti-LC3B | Abcam, CA, USA | ab48394 | |
| anti-p62 | Abcam | ab91526 | |
| anti-β-actin antibody | Abcam | ab8227 | |
| Binding buffer | BD Biosciences | 556454 | |
| DMEM | Thermo Fisher Scientific, Waltham, MA, USA | 11965092 | |
| EVOS XL Core microscope | Thermo Fisher Scientific | AMEX1000 | |
| Goat anti-rabbit H&L HRP-conjugated secondary antibodies | Abcam | ab205718 | |
| Leukemiavirus reverse transcriptase | Clontech | 639574 | |
| Male Wistar rats | Shanghai SLAC Laboratory Animal Co., Ltd | 200–240 g, 8 weeks | |
| Oligo (dT)18 Primer | TaKaRa | 3806 | |
| Primers | Shanghai Sangon Biotech | Synthesized primers for IL-1β, IL-6 and IL-10 | |
| RIPA Lysis Buffer | Thermo Fisher Scientific | 20-188 | |
| RNA extraction kit (Trizol) | TaKaRa | 9108Q | |
| StepOne Realtime PCR System | Thermo Fisher Scientific | 4376357 | |
| TUNEL assay kit | Thermo Fisher Scientific | C10245 |
参考文献
- Cho, S. B., Kim, S. C., Chung, M. G. Identification of novel population clusters with different susceptibilities to type 2 diabetes and their impact on the prediction of diabetes. Scientific Reports. 9 (1), 3329 (2019).
- Diamant, A. L., Babey, S. H., Hastert, T. A., Brown, E. R. Diabetes: the growing epidemic. Policy Brief (UCLA Center for Health Policy Research. , 1-12 (2007).
- Zhang, H., Qi, R., Zeng, Y., Tsao, R., Mine, Y. Chinese sweet leaf tea (Rubus suavissimus) mitigates LPS-induced low-grade chronic inflammation and reduces the risk of metabolic disorders in a C57BL/6J mouse model. Journal of Agricultural and Food Chemistry. 68 (1), 138-146 (2019).
- Tian, S., Wang, M., Liu, C., Zhao, H., Zhao, B. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin Signalling pathway. BMC Complementary and Alternative Medicine. 19 (1), 326 (2019).
- Butler, A. E., Janson, J., Soeller, W. C., Butler, P. C. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 52 (9), 2304-2314 (2003).
- Ramirez-Farias, C., et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. British Journal of Nutrition. 101 (4), 541-550 (2009).
- Zhang, Y., et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. The Journal of Clinical Endocrinology and Metabolism. 93 (7), 2559-2565 (2008).
- Turnbaugh, P. J., Backhed, F., Fulton, L., Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host & Microbe. 3 (4), 213-223 (2008).
- Jamil, S., et al. Angiopoietin-like 4 enhances the proliferation and migration of tendon fibroblasts. Medicine & Science in Sports & Exercise. 49 (9), 1769-1777 (2017).
- Bohm, S., Mersmann, F., Tettke, M., Kraft, M., Arampatzis, A. Human achilles tendon plasticity in response to cyclic strain: effect of rate and duration. Journal of Experimental Biology. 217, 4010-4017 (2014).
- Mousavizadeh, R., et al. Cyclic strain alters the expression and release of angiogenic factors by human tendon cells. PLoS One. 9 (5), 97356 (2014).
- Dong, H., Zhao, Y., Zhao, L., Lu, F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Medica. 79 (6), 437-446 (2013).
- Dong, H., Wang, N., Zhao, L., Lu, F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2012, 591654 (2012).
- Pirillo, A., Catapano, A. L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 243 (2), 449-461 (2015).
- Sun, S. F., et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clinical and Experimental Pharmacology and Physiology. 42 (6), 662-670 (2015).
- Zhai, J., et al. Berberine protects against diabetic retinopathy by inhibiting cell apoptosis via deactivation of the NFkappaB signaling pathway. Molecular Medicine Reports. 22 (5), 4227-4235 (2020).
- Zhang, J. H., et al. Effects of Berberine on diabetes and cognitive impairment in an animal model: The mechanisms of action. The American Journal of Chinese Medicine. 49 (6), 1399-1415 (2021).
- Jandrey, E. H. F., et al. A key pathway to cancer resilience: The role of autophagy in glioblastomas. Frontiers in Oncology. 11, 652133 (2021).
- Kroemer, G., Levine, B. Autophagic cell death: the story of a misnomer. Nature Reviews Molecular Cell Biology. 9 (12), 1004-1010 (2008).
- Hoshino, A., et al. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic beta-cell function in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 111 (8), 3116-3121 (2014).
- Jung, H. S., et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metabolism. 8 (4), 318-324 (2008).
- Zhang, M., et al. Highly bioavailable berberine formulation ameliorates diabetic nephropathy through the inhibition of glomerular mesangial matrix expansion and the activation of autophagy. European Journal of Pharmacology. 873, 172955 (2020).
- Jia, Y., Xu, B., Xu, J. Effects of type 2 diabetes mellitus on the pharmacokinetics of berberine in rats. Pharmaceutical Biology. 55 (1), 510-515 (2017).
- Sakamoto, K., et al. Involvement of Na+/Ca2+ exchanger in migration and contraction of rat cultured tendon fibroblasts. Journal of Physiology. 587, 5345-5359 (2009).
- Mendias, C. L., Gumucio, J. P., Lynch, E. B. Mechanical loading and TGF-beta change the expression of multiple miRNAs in tendon fibroblasts. Journal of Applied Physiology. 113 (1), 56-62 (2012).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25 (4), 402-408 (2001).
- Oliver, T. I., Mutluoglu, M. Diabetic Foot Ulcer. Treasure Island (FL): StatPearls Publishing. , (2020).
- Zeng, T., et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clinical science. 133 (9), (2019).
- Sardone, F., et al. Collagen VI-NG2 axis in human tendon fibroblasts under conditions mimicking injury response. Matrix Biology. 55, 90-105 (2016).
- de Oliveira, A. R., et al. Effect of photobiomodulation and exercise on early remodeling of the Achilles tendon in streptozotocin-induced diabetic rats. PLoS One. 14 (2), 0211643 (2019).
- Wu, Y. F., et al. High glucose alters tendon homeostasis through downregulation of the AMPK/Egr1 pathway. Scientific Reports. 7, 44199 (2017).
- Garcia-Bailo, B., et al. E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stress. Biologics. 5, 7-19 (2011).
- Hudgens, J. L., et al. Platelet-rich plasma activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. American Journal of Sports Medicine. 44 (8), 1931-1940 (2016).
- Chen, H., et al. Berberine attenuates apoptosis in rat retinal Muller cells stimulated with high glucose via enhancing autophagy and the AMPK/mTOR signaling. Biomedicine & Pharmacotherapy. 108, 1201-1207 (2018).
- Li, G., et al. Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. American Journal of Physiology-Heart and Circulatory Physiology. 315 (4), 802-813 (2018).
- Yerra, V. G., Kalvala, A. K., Sherkhane, B., Areti, A., Kumar, A. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology. 131, 256-270 (2018).
- Zhou, G., Yan, M., Guo, G., Tong, N. Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-gamma, and AMPK expressions. Dose Response. 17 (3), 1559325819862449 (2019).
- Zhu, L., Han, J., Yuan, R., Xue, L., Pang, W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-kappaB pathway. Biological Research. 51 (1), 9 (2018).
- Han, Y., et al. Pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evidence-Based Complementary and Alternative Medicine. 2021, 9987097 (2021).
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacological Research. 155, 104722 (2020).

.
