Solution Blow Spinning of Polymeric Nano-Composite Fibers for Personal Protective Equipment
概要
The primary goal of this study is to describe a protocol to prepare polymeric fiber mats with consistent morphology via solution blow spinning (SBS). We aim to use SBS to develop novel, tunable, flexible polymeric fiber nanocomposites for various applications, including protective materials, by incorporating nanoparticles in a polymer-elastomer matrix.
Abstract
Light-weight, protective armor systems typically consist of high modulus (>109 MPa) and high-strength polymeric fibers held in place with an elastic resin material (binder) to form a non-woven, unidirectional laminate. While significant efforts have focused on improving the mechanical properties of the high-strength fibers, little work has been undertaken to improve the properties of the binder materials. To improve the performance of these elastomeric polymer binders, a relatively new and simple fabrication process, known as solution blow spinning, was used. This technique is capable of producing sheets or webs of fibers with average diameters ranging from the nanoscale to the microscale. To achieve this, a solution blow spinning (SBS) apparatus has been designed and built in the laboratory to fabricate non-woven fiber mats from polymer elastomer solutions.
In this study, a commonly used binder material, a styrene-butadiene-styrene block-co-polymer dissolved in tetrahydrofuran, was used to produce nanocomposite fiber mats by adding metallic nanoparticles (NPs), such as iron oxide NPs, that were encapsulated with silicon oil and thus incorporated in the fibers formed via the SBS process. The protocol described in this work will discuss the effects of the various critical parameters involved in the SBS process, including the polymer molar mass, the selection of the thermodynamically appropriate solvent, the polymer concentration in solution, and the carrier gas pressure to assist others in performing similar experiments, as well as provide guidance to optimize the configuration of the experimental setup. The structural integrity and morphology of the resultant non-woven fiber mats were examined using scanning electron microscopy (SEM) and elemental X-ray analysis via energy-dispersive X-ray spectroscopy (EDS). The goal of this study is to evaluate the effects of the various experimental parameters and material selections to optimize the structure and morphology of the SBS fiber mats.
Introduction
Many light-weight, ballistic, protective armor systems are currently constructed using high-modulus and high-strength polymeric fibers, such as oriented, ultra-high molar mass polyethylene fibers or aramids, which provide outstanding ballistic resistance1,2. These fibers are used in combination with an elastic resin material (binder) that can penetrate to the filament level and secure the fibers in a 0°/90° configuration to form a non-woven, unidirectional laminate. The percentage of the polymer elastomer resin (binder) should not exceed 13% of the total weight of the unidirectional laminate to maintain the structural integrity and antiballistic properties of the laminate structure3,4. The binder is a very important component of the armor as it keeps the high-strength fibers properly oriented and tightly packed within each laminate layer3. Elastomer materials commonly used as binders in body armor applications have very low tensile modulus (e.g., ~17.2 MPa at ~23 °C), low glass transition temperature (preferably below -50 °C), very high elongation at break (as high as 300%) and must demonstrate excellent adhesive properties5.
To improve the performance of these polymer elastomers, SBS was performed to create fibrous elastomer materials that can be used as binders in body armor applications. SBS is a relatively new, versatile technique allowing the use of different polymer/solvent systems and the creation of different end products6,7,8,9,10,11,12,13. This simple process involves the rapid (10x the rate of electrospinning) deposition of conformal fibers onto both planar and nonplanar substrates to fabricate sheets or webs of fibers that encompass nano and micro length scales14,15,16,17,18. SBS materials have numerous applications in medical products, air filters, protective equipment, sensors, optical electronics, and catalysts14,19,20. Developing small diameter fibers can drastically increase the surface area to volume ratio, which is very important for several applications, especially in the personal protective equipment field. The diameter and morphology of the fibers generated by SBS depend on the molar mass of the polymer, polymer concentration in the solution, viscosity of the solution, polymer solution flow rate, gas pressure, working distance, and diameter of the spray nozzle14,15,17.
An important characteristic of the SBS apparatus is the spray nozzle consisting of an inner and a concentric outer nozzle. The polymer dissolved in a volatile solvent is pumped through the inner nozzle while a pressurized gas flows through the outer nozzle. The high-velocity gas exiting the outer nozzle induces shearing of the polymer solution flowing through the inner nozzle. This forces the solution to form a conical shape when exiting the spray nozzle. When the surface tension at the tip of the cone is overcome, a fine stream of polymer solution is ejected, and the solvent rapidly evaporates causing polymer strands to coalesce and deposit as polymer fibers. The formation of a fibrous structure, as solvent evaporates, strongly depends on the polymer molar mass and the solution concentration. Fibers are formed by chain entanglement, when polymer chains in solution begin to overlap at a concentration known as the critical overlap concentration (c*). Therefore, it is necessary to work with polymer solutions above the c* of the polymer/solvent system selected. Also, an easy strategy to attain this is to choose polymers with relatively high molar mass. Polymers with higher molar mass have increased polymer relaxation times, which is directly related to an increase in the formation of fibrous structures, as described in the literature21. As many of the parameters used in SBS are strongly correlated, the goal of this work is to provide guidance to develop tunable, and flexible polymeric fiber nanocomposites to be used as alternatives for typical binder materials found in body armor applications by incorporating nanoparticles in the fibrous polymer-elastomer matrix.
Protocol
NOTE: Details related to the equipment, instrumentation, and chemicals used in this section can be found in the Table of Materials. This entire protocol should first be reviewed and approved by the institutional safety department/personnel to ensure procedures and processes specific to the institution are adhered to.
1. Preparation of polymer solution using the appropriate solvent
NOTE: Consult manufacturer/supplier safety data sheets and the institution's safety department/personnel regarding proper personal protective equipment (PPE) to use with each chemical/material.
- Use a clean small laboratory spatula, and transfer the desired amount (e.g., ~2 g) of dry polymer (poly(styrene-butadiene-styrene)) into a clean, empty, 20 mL borosilicate glass vial. Seal the vial, and store under ambient laboratory conditions.
NOTE: The selected concentration for poly(styrene-butadiene-styrene) in tetrahydrofuran (THF) was approximately 200 mg/mL. This concentration is used as an example throughout this protocol; the optimal concentration will depend on the polymer/solvent system used. - Transfer the borosilicate glass vial containing the polymer sample into a chemical fume hood, and pipette 10 mL ± 0.1 mL of the desired solvent, in this case THF, into the vial to achieve the desired concentration of nominally 200 mg/mL.
- Seal the solvent (THF) container, and transfer it to the storage cabinet. Cap the borosilicate glass vial containing the polymer/solvent sample with the provided lid, and carefully mount it on a mixer/rotator.
- Agitate the mixture at room temperature using a rotator at 70 rpm until the polymer fully dissolves in the solvent.
NOTE: The solution appears clear and transparent after approximately 60 min, denoting complete polymer dissolution. - Transfer the solution into a dissolved gas analysis (DGA) borosilicate glass syringe for SBS.
NOTE: Polymer solutions can be stored and used for up to 72 h, provided that the borosilicate glass vial is securely capped, and the opening is wrapped using a paraffin wax film. However, the solutions must be agitated again before performing SBS.
2. Determination of critical overlap polymer concentration by viscosity measurement
NOTE: This step is provided here to determine the critical overlap polymer concentration, which is an important parameter that affects the overall fiber quality and morphology after SBS. See the representative results and discussion sections for details.
- Prepare eight nominal concentrations (1 mg/mL, 3 mg/mL, 5 mg/mL, 10 mg/mL, 20 mg/mL, 30 mg/mL, 40 mg/mL, 50 mg/mL) of the polymer solution in THF with an approximate volume of 10 mL. Follow the same procedure as in steps 1.1 and 1.2 to prepare the solutions.
- Prepare the rheometer for measurements.
NOTE: Routine calibration and verification checks for torque, normal force, and phase angle should be performed on the rheometer prior to the following setup procedure.- Install the environmental control device on the rheometer for temperature control.
- Install the rheometer geometry, i.e., recessed concentric cylinders on the rheometer. First, insert and install the lower geometry (cup) into the environmental control device and then the upper geometry (bob) on the transducer shaft.
- Tare normal force and torque using the instrument touch screen. Zero the geometry gap using the gap control function of the rheometer software. Raise the stage to provide enough space for sample loading.
- Load the polymer solution into the cup using a high-quality, disposable borosilicate glass pipette (minimum sample volume for the geometry ~7 mL). Set the gap to the operating gap (3.6 mm) for the measurement.
- Perform a shear rate sweep test from about 10 s-1 to 100 s-1 at approximately 25 °C. Enable the steady-state sensing function in the rheometer software.
- Export the results table, and calculate the average value of the steady-shear viscosities.
- Plot the averaged viscosity values as a function of polymer concentration.
3. Preparation of the polymer solution/nanoparticle dispersion
NOTE: To prepare a polymer solution with added nanoparticles (NPs), work inside a nano-enclosure (high-efficiency-particulate-air-filtered) hood.
- Use a clean, small laboratory spatula, and weigh the required amount (e.g., ~0.01 g) of dry NP powder, e.g., iron oxide (Fe3O4) NPs, into a clean 20 mL borosilicate glass vial.
- Add the desired volume (e.g., nominally 10 mL) of solvent (e.g., THF) using a disposable borosilicate glass pipette, and cap the borosilicate glass vial containing the NPs/solvent mixture using the provided lid.
- Transfer the sample to a vortex mixer, and agitate thoroughly at room temperature at 3,000 rpm until the NPs are no longer visible at the bottom of the vial. Immediately transfer the vial with the sample to a bath sonicator to ensure full dispersion of the nanoparticles. To prevent sample from heating, sonicate the dispersion in ~30 min intervals, waiting for 2-5 min between each sonication step.
- Next, working inside a chemical hood, weigh and add the desired amount (e.g., ~2 g) of polymer (e.g., styrene-butadiene-styrene block-co-polymer) into the NP dispersion. Seal the borosilicate glass vial with the provided lid, and mount it securely on a rotator for mixing at 70 rpm at room temperature.
- Mix the polymer/NPs/solvent sample thoroughly for approximately 60 min, or until the polymer is fully dissolved.
NOTE: After mixing, the sample appears as a viscous liquid with uniformly dispersed NPs, and no large aggregates or precipitates are visible. - Finally, transfer the mixture into a DGA borosilicate glass syringe for SBS.
NOTE: It is not recommended to store the polymer NP solutions prior to SBS due to potential agglomeration or destabilization of the dispersion.
4. Solution blow spinning process (SBS)
NOTE: Suggested PPE for this process includes protective goggles, laboratory coat, and nitrile gloves; these should be donned before setting up the SBS apparatus. The setup and process should be performed inside a chemical hood. The SBS apparatus consists of a commercial airbrush unit equipped with a 0.3 mm inner nozzle (for the polymer solution) and a 1 mm head opening (for the gas), a syringe pump system, a collector, a pressurized nitrogen (N2) gas cylinder, and an aluminum enclosure. The inner nozzle protrudes approximately 0.5 mm from the head opening of the airbrush. Details on the SBS setup are given in Figure 1.
- First, adjust the height and angle of the airbrush to align with the center of the selected substrate (glass microscope slide) attached to the collector, and secure it in place. Make sure the gas cylinder is properly secured to its wall mount. Then, connect the gas inlet of the airbrush to the N2 pressurized gas cylinder.
- Turn on the main valve on the gas cylinder, and slowly adjust the pressure using the attacjed gas regulator valve while monitoring the pressure gauge to achieve the desired flow. Ensure there is free unobstructed flow through the system, and listen carefully for any potential gas leaks at the connection points. Use a soap and water solution to further investigate potential leaks, and if necessary, apply polytetrafluoroethylene (PTFE) tape to the fittings to eliminate any leaks. When the gas flow is adjusted properly, close the main valve on the gas cylinder to stop the gas flow.
- Secure the substrate on the collector using the equipped vice. Adjust the height of the collector to align perpendicular to the spray direction and pattern of the airbrush so that material will be deposited onto the substrate.
- Next, slide the collector to its furthest position away from the airbrush nozzle to help with identifying the optimal working distance (separation between nozzle and substrate) in the following steps.
- Working inside the chemical hood, carefully transfer the prepared polymer/NPs/solvent mixture from the borosilicate glass vial to a 10 mL DGA borosilicate glass syringe equipped with a stainless-steel needle.
- Remove any air bubbles from the sample by holding the syringe with the needle pointing up, tapping the syringe gently and slowly depressing the plunger to displace any excess air. Detach the needle, and attach the syringe to the syringe-pump unit. Secure the syringe, and connect the PTFE tubing coming from the outlet of the syringe to the appropriate inlet on the airbrush.
- Next, select the desired injection rate from the syringe-pump unit menu (e.g., 0.5 mL/min), and slowly open the main valve on the N2 gas cylinder to allow N2 to flow through the airbrush. Immediately start the syringe-pump unit to dispense the polymer/NPs/solvent mixture, and initiate the spraying process.
- Carefully observe the spraying pattern at the spray nozzle, and ensure that no clogs or partial clogs are present. Incrementally increase or decrease the injection rate until the solution is spraying freely.
NOTE: Very low or high injection rates are prone to clogging. The optimal injection rate is a function of the solution's viscosity and may need to be adjusted for high or low polymer solution's concentrations. - Next, adjust the position of the collector to the desired working distance for the polymer/solvent system used to allow solvent evaporation by sliding it towards the airbrush until material is deposited on the substrate.
NOTE: If the collector is too close to the airbrush spray nozzle, insufficient evaporation time will result in depositing liquid polymer solution onto the substrate. If the collector is too far away, very limited or no material will be deposited onto the substrate. For poly(styrene-butadiene-styrene) solutions in THF, the appropriate working distance is between 8 cm and 12 cm. - When the desired amount of material is deposited on the substrate, stop the syringe-pump unit first, and then immediately close the main valve on the N2 gas cylinder.
5. Analysis of SBS fiber mats by SEM
- Use a sputter coater to coat the fiber mats with a conductive material such as Au/Pd to mitigate surface charging effects under the electron beam.
NOTE: A coating thickness of 4-5 nm will suffice. - Load the fiber mat samples into an SEM, and image them using an accelerating voltage of 2-5 kV and a current of 0.1-0.2 nA. Apply charge neutralization settings to counter charging effects where necessary.
- Use a secondary electron detector, or a backscattered electron detector, to capture different features of the fiber materials.
- Use an energy-dispersive (EDS) detector to separate the characteristic X-rays of different elements into an energy spectrum that will allow determination of the presence of iron (Fe), indicative of iron oxide NPs embedded within the polymeric fiber mats.
Representative Results
In this study, non-woven fiber mats consisting of poly(styrene-butadiene-styrene) fibers in the nano- and micro-scale, were synthesized with and without the presence of iron oxide NPs. To form fibers, the SBS parameters must be carefully selected for the polymer/solvent system used. The molar mass of the dissolved polymer and the solution concentration are critical in controlling the morphology of the structures produced by the SBS process. In this study, a poly(styrene-butadiene-styrene) block-co-polymer (styrene 30 wt. %) was used with a molar mass of approximately 185,000 g/mol and a density of 0.94 g/mL at 25 °C. Multiple studies have examined the effects of polymer molar mass, demonstrating that a higher molar mass favors chain entanglement in solution and drastically increases its viscosity, resulting in fiber formation via the SBS technique21. In addition, previous studies have shown that polymer concentrations in a good solvent (as defined by Flory22) well above the critical overlap concentration (c >> c*), also known as the entanglement concentration (Ce ~ 10c*), will result in fiber formation with minor or no bead formation21,23.
This phenomenon is again governed by the interactions between the entangled polymer chains in solution. Entanglement of the molecules in solution above the c* exponentially increases the viscosity of the solution, therefore overcoming the inertial capillary forces and suppressing the breakup of the polymer jet. Destabilization of the polymer jet after shearing off the polymer solution stream from the nozzle will lead to undesirable "bead" formation if the selected concentration for the SBS experiment is too low. In this study, the critical overlap concentration of the poly(styrene-butadiene-styrene) block-co-polymer in THF was first estimated using the following equation for random polymer coils in a good solvent24:
c* ≈ 3Mw/(4πNARg3) (1)
In equation (1) above, NA, Mw, and Rg are the Avogadro's number, the molar mass of the polymer, and the radius of gyration of the polymer, respectively. This equation estimated the c* of the polymer in solution to be ~8.96 mg/mL. Eight polymer solutions were prepared with different concentrations and their viscosity was studied as a function of concentration. For most polymers, the behavior of their solution's viscosity in a good solvent is linear only at low concentrations.
As polymer concentration increases, the viscosity rises exponentially, and the critical overlap concentration corresponds to the value at which the dissolved polymer coils start to overlap each other and cause entanglement. At that critical concentration, a polymer solution transitions from a dilute to a semi-dilute regime25. The results of the polymer solution's viscosity as a function concentration are shown in Figure 2, and the value of the experimentally estimated c* is ~9.28 mg/mL. The calculated and experimentally predicted values of c* are similar, which is ~10 mg/mL. Therefore, polymer concentration values greater than 10c* (c ≥ 100 mg/mL) were selected to use for the SBS process, to be in the entanglement concentration regime23. At these higher concentrations, the SBS apparatus is capable of consistently producing non-woven fibers with desired diameters and morphology. Figure 3 shows the structure of the developed fiber mats and the morphology of the fibers at a polymer concentration of ~200 mg/mL, N2 gas pressure of approximately 207 kPa, a working distance of nominally 8 cm, and a polymer solution injection rate of ~0.5 mL/min.
The electron micrograph in Figure 3A shows the morphology of the non-woven fiber mat at low magnification. The fiber mat sample consists of primarily individual and cylindrically shaped fibers with minimal polymer beads or polymer welding present. At higher magnification (Figure 3B), it is apparent that the fibers formed are smooth and round, with very similar diameters in the nano scale (diameter range from 100 nm to 600 nm). Individual fibers, as well as some bundle of fibers consisting of 2, 3, and sometimes 4 individual fibers are observed. Finally, the higher magnification images confirm the absence of polymer beads ("beads-on-a-string") or polymer welding under these SBS conditions. To better understand this specific polymer/solvent system and the effect of polymer concentration on the fiber mats produced, the structure and morphology of fiber mat samples sprayed at various concentrations were examined. Significant differences in the fiber mats produced were observed as the polymer concentration increased from approximately 100 mg/mL to 120 mg/mL, 150 mg/mL, and 200 mg/mL, respectively, as seen in Figure 4. The SEM micrographs show a clear transition from fibers that exhibit the undesired "beads-on-a-string" morphology with numerous bundles of fibers present at lower concentrations, near the critical overlap concentration (c* ~10 mg/mL), to the formation of pristine and morphologically smooth fibers at concentrations well above c* (e.g., 200 mg/mL).
Furthermore, as mentioned previously, the gas pressure is another process variable that can influence the morphology and diameter of the produced fibers, although to a much lesser extent than polymer molar mass and concentration. Figure 5 demonstrates the effects of gas pressure, indicating the presence of fibers with decreasing diameter as gas pressure increased from ~138 kPa to ~345 kPa; however, the presence of large polymer beads and welded fibers also increased. Prior work has also demonstrated that very high gas pressures will induce undesirable fiber and polymer welding17,19. This effect could be a result of a more significant decrease in temperature at the spray nozzle when higher gas flow rates are used, due to Joule expansion of the gas. The temperature decrease is proportional to the volumetric expansion of the gas, which in turn, can cause poor solvent evaporation and fiber welding17,19,26. In the current study, based on various SBS parameters and SEM imaging, the optimal polymer concentration and carrier gas pressure for the polymer/solvent system were determined to be 200 mg/mL and 207 kPa.
This combination can consistently produce pristine, smooth, individual fibers in the nano scale (diameter of ~100 nm to 600 nm) without the presence of beads or fiber welding, as shown in Figure 3. It is useful to note that the nitrogen gas was fed to the SBS sprayer through a PTFE tube with an inner diameter of 0.238 cm and length of 2.134 m. At the optimal nitrogen pressure of 207 kPa and approximately 20 °C, the N2 gas density is 0.00215 kg/L, its dynamic viscosity is 1.76 x 10-5 Pa·s, and its approximate velocity is 0.871 m/s with a Reynold's number of 147, indicating a laminar flow. After identifying the best conditions for the SBS parameters in this spray setup for poly(styrene-butadiene-styrene) in THF, the capability of the technique to produce polymer elastomer nanocomposite fiber mats was investigated by dispensing iron oxide NPs in the polymer solution at a mass fraction of χnp = 0.001. This mass fraction was determined to be the highest attainable before destabilization of the NP dispersion was observed. As the NP dispersions were not stable above χnp = 0.001, no dispersions were sprayed at NP mass fractions above this value. Nanoparticle agglomeration phenomena are to be expected, which can later affect the quality of the fibers produced (irregular fiber morphology and diameters) and result in a non-uniform dispersion of the NPs within the fiber material.
It is important to note that after sonication, the iron oxide NP/polymer dispersions at mass fractions equal to 0.001 were stable for approximately 2 hours; therefore, it is recommended to use them immediately after mixing for optimal results. If the dispersions are left unmixed for more than a few hours, it is recommended to sonicate the samples again before beginning SBS. The NPs used in this study, in the form of dry powder, were coated by the manufacturer with silicon oil, which renders them easily dispersible in various organic solvents, including THF. The fiber mats produced were evaluated using backscattered electron (BSE) analysis and EDS in an SEM, and the results demonstrate the presence of iron oxide NPs within the polymer fibers. A representative electron micrograph collected via a BSE detector is shown in Figure 6A. The iron oxide particles (circled in red) can be easily identified in the fibers due to their brighter contrast from the surrounding polymeric fiber material using a BSE detector, as iron is a much heavier element than carbon. In Figure 6C, EDS elemental analysis of the same sample indicates the presence of iron (marked red) at the brighter contrast locations where the iron oxide NPs reside, further validating their presence within the fibers. It is worth noting that the structure and morphology of the fiber mats were not significantly affected by the presence of the iron oxide NPs.
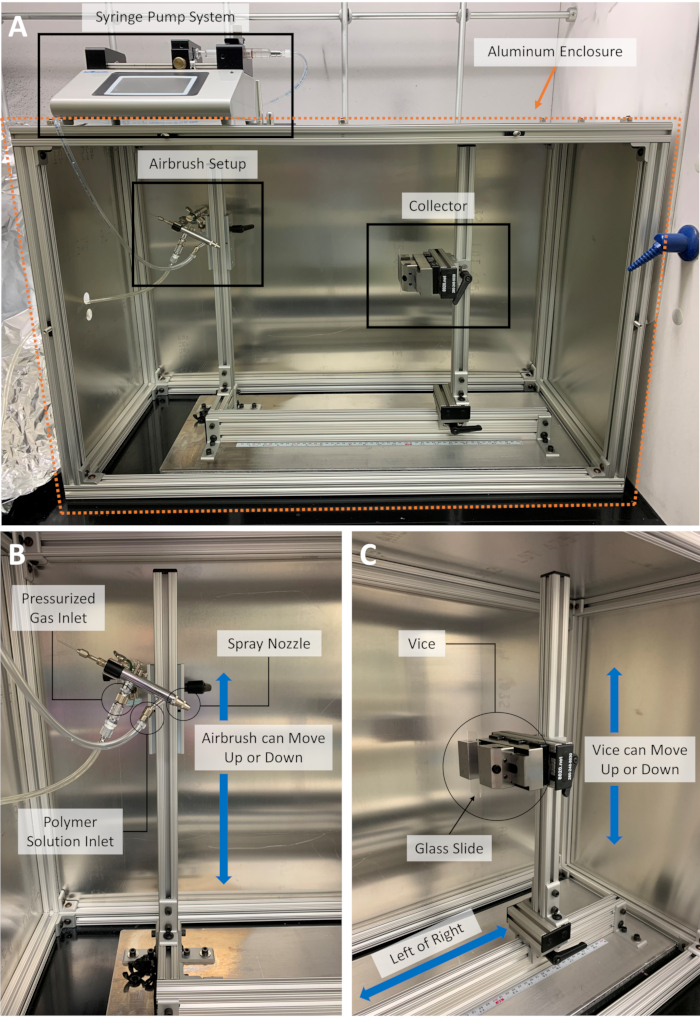
Figure 1: The solution blow spinning apparatus. (A) The apparatus comprises a syringe pump system, an airbrush setup, a collector, an aluminum enclosure, and a nitrogen gas cylinder (not shown); details on the (B) airbrush setup and (C) substrate holder are shown. Please click here to view a larger version of this figure.
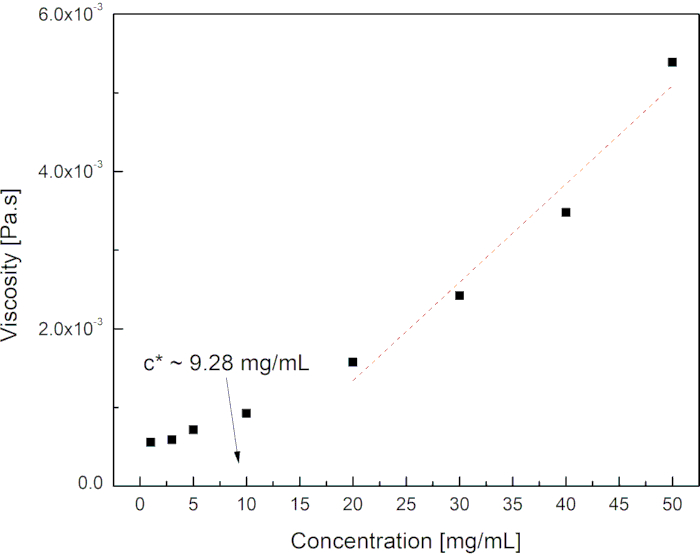
Figure 2: Viscosity of polymer solutions as a function of polymer concentration. The critical overlap concentration (c*) is estimated by the onset of the power law behavior of the viscosity as indicated by the arrow in the graph. Please click here to view a larger version of this figure.

Figure 3: Scanning electron microscopy (SEM) images of poly(styrene-butadiene-styrene) fibers formed via the solution blow spinning (SBS) apparatus. (A) Low-magnification image, and (B) higher-magnification image of the same sample. Scale bar for A = 1 mm; scale bar for B = 40 µm. Please click here to view a larger version of this figure.
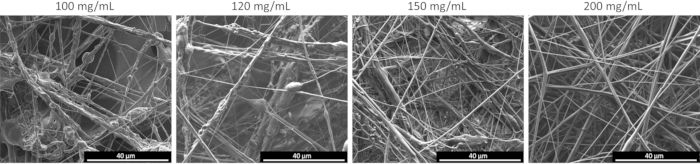
Figure 4: SEM micrographs of poly(styrene-butadiene-styrene) solutions sprayed using the SBS apparatus at increasing polymer concentration in solution. Polymer concentration increases from left to right. Scale bars = 40 µm. Abbreviations: SEM = scanning electron microscopy; SBS = solution blow spinning. Please click here to view a larger version of this figure.
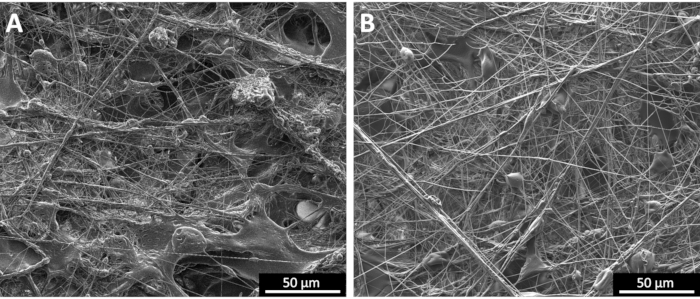
Figure 5: SEM micrographs of poly(styrene-butadiene-styrene) solutions sprayed using the SBS apparatus. (A) High gas pressure of 345 kPa and (B) low gas pressure of 138 KPa. Scale bars = 50 µm. Please click here to view a larger version of this figure.

Figure 6: Backscattered electron micrograph of solution blow spun poly(styrene-butadiene-styrene) fibers. (A) Infused iron oxide (Fe3O4) nanoparticles circled in red; scale bar = 10 µm. (B) Enlargement of the yellow highlighted area at the same magnification. (C) Energy-dispersive X-ray spectroscopy of the enlarged area, indicating the presence of iron (elemental analysis; Fe stained red) within the fibers. Scale bars (B,C) = 4 µm. Please click here to view a larger version of this figure.
Discussion
The method described herein provides a protocol for producing polymer elastomer nanocomposite fiber mats via a relatively new technique known as solution blow spinning. This technique allows the fabrication of fibers in the nanoscale and has several advantages over other well-established techniques, such as the electrospinning process, as it can be carried out under atmospheric pressure and room temperature27. Furthermore, SBS is not highly susceptible to local environmental changes (temperature or humidity) and does not require harsh or toxic chemicals, nor a high voltage gradient, which is beneficial when working with biological systems17,19,28,29. Finally, the solution deposition rates of the SBS process are roughly 10 times faster than those of electrospinning, covering larger areas in less time and promising ease of process scale-up14,17.
To obtain desirable results from this technique and the protocol described herein, the operator needs to carefully select the materials and have control over certain important parameters, such as the polymer molar mass and concentration, as well as the carrier gas pressure. The selection of the desired polymer will dictate the solvent that should be used. The solvent needs to be volatile at ambient conditions and must be a good solvent for the selected polymer to be dissolved at concentrations equal or greater than the c*, which can be achieved by examining the Hildebrand solubility parameters (δ).
In this study, it was recommended to use a solvent with solubility parameters between 7.7 and 9.4 for the poly(styrene-butadiene-styrene) block-co-polymer used. Therefore, THF was as a solvent with a Hildebrand solubility parameter of δ = 9.130. Next, the molar mass of the polymer (~180,000 g/mol) was judiciously selected to be high enough to promote polymer chain entanglements and achieve solutions with sufficiently high viscosities that were previously shown to favor fiber formation via the SBS technique21. The structure and morphology of the produced fibers vary not only with increasing molar mass, but also with polymer concentration.
The results of this study have shown that concentrations well above the critical overlap concentration (c >> c*) result in fibers with optimal size and morphology, which is also well supported by the literature14,17,21. Then, the effect of the carrier gas pressure was investigated; extremely low or high values were found to negatively impact the fiber morphology. Smaller fiber diameters were observed with increasing gas pressure values, along with the formation of fiber welding at very high pressures. Therefore, gas pressures of ~207 kPa are desirable for this polymer/solvent system and this SBS setup. These changes in the morphology of the produced fibers as a result of the gas pressure were also observed in a study by Medeiros et al.14
Finally, other parameters including the working distance, polymer solution injection rate, and spraying time were kept constant at values that allowed proper solvent evaporation, no clogging issues at the spray nozzle, and same amounts of deposited material, respectively. Ultimately, fibrous materials developed via the SBS process have numerous applications in various field including medical products, air filters, protective equipment, sensors, optical electronics, and catalysts14,19,20. Thus, the ultimate goal of this research is to use the SBS technique described herein to create tunable polymer elastomer nanocomposites, and apply them to advance various fields, such as the personal protective equipment field, which involve the use of novel multifunctional materials.
開示
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Mr. Dwight D. Barry for his important contributions for fabrication of the solution blow spinning apparatus. Zois Tsinas and Ran Tao would like to acknowledge funding from the National Institute of Standards and Technology under Awards # 70NANB20H007 and # 70NANB15H112, respectively.
Materials
| 45 MM Toolmaker Vise | Tormach Inc. | 32547 | To secure substrate onto the collector |
| ARES-G2 Rheometer | TA Instruments | 401000.501 | Rheometer |
| Branson Ultrasonics M Series – Ultrasonic Cleaning Bath | Fisher Scientific | 15-336-100 | To disperse nanoparticles |
| Cadence Science Micro-Mate Interchangeable Syringe | Fisher Scientific | 14-825-2A | Glass Syringe 5mL in 1/5mL, Luer Lock Tip |
| Chemical hood | Any company | ||
| Corning – Disposable Pasteur Glass Pipette | Sigma Aldrich | CLS7095D5X-200EA | Non-Sterile |
| DWK Life Sciences Wheaton – Glass Scintillation Vial | Fisher Scientific | 03-341-25G | 20 mL with cap |
| FEI Quanta 200 Scanning Electron Microscope (SEM) | FEI | For imaging samples | |
| Iron Oxide Nanopowder/Nanoparticles | US Research Nanomaterials, inc. | US3320 | Fe3O4, 98%, 20-3- nm, Silicon oil Coated |
| KD Scientific Legato 100 Single-Syringe Pump | Sigma Aldrich | Z401358-1EA | Single syringe infusion pump |
| Master Airbrush – Model S68 | TCP Global | MAS S68 | Nozzle/needle diameter: 0.35 mm |
| Mettler Toledo AB265-S/FACT Scale | Cole-Parmer Scientific | EW-11333-14 | For weighing polymer and Nanoparticles |
| N2 Gas Regulator | Any company | ||
| Nanoenclosure | Any company | ||
| Optical Microscopy Glass Slides | Fisher Scientific | 12-550-A3 | Used as a substrate for fiber mat deposition |
| OSP Slotted Bob, 33 mm | TA Instruments | 402796.902 | Bob, upper geometry |
| OSP Slotted Double Gap Cup, 34 mm | TA Instruments | 402782.901 | Double wall cup, lower geometry |
| Oxford BenchMate Digital Vortex Mixer | Pipette | VM-D | Rated up to 4,200 rpm, for mixing solutions |
| Oxford Benchmate Tube Roller | Pipette | OTR-24DR | Sample mixer/rotator |
| Polystyrene-block-polybutadiene-block-polystyrene | Sigma Aldrich | 432490-1KG | styrene 30 wt. %, Mw ~ 185,000 g/mol |
| SEM Pin Stub Specimen Mount | Ted Pella Inc. | 16119 | 18 mm diameter x 8 mm height |
| Spatula | VWR | 82027-532 | To load test materials |
| Tetrahydrofuran (THF) | Fisher Scientific | T425-1 | solvent, HPLC grade |
| TRIOS | TA Instruments | v4.3.1.39215 | Rheometer software |
参考文献
- Lee, B. L., et al. Penetration failure mechanisms of armor-grade fiber composites under impact. Journal of Composite Materials. 35 (18), 1605-1633 (2001).
- Prevorsek, D. C., Kwon, Y. D., Chin, H. B. Analysis of the temperature rise in the projectile and extended chain polyethylene fiber composite armor during ballistic impact and penetration. Polymer Engineering and Science. 34 (2), 141-152 (1994).
- Park, A. D., Park, D., No Park, A. J. . U.S. Patent. , (2006).
- No Park, A. D. . U.S. Patent. , (1995).
- Harpell, G. A., Prevorsek, D. C., Li, H. L. Flexible multi-layered armor. Patent No. WO/1989. , (1989).
- Cena, C., et al. BSCCO superconductor micro/nanofibers produced by solution blow-spinning technique. Ceramics International. 43 (10), 7663-7667 (2017).
- Miller, C. L., Stafford, G., Sigmon, N., Gilmore, J. A. Conductive nonwoven carbon nanotube-PLA composite nanofibers towards wound sensors via solution blow spinning. IEEE Transactions on Nanobioscience. 18 (2), 244-247 (2019).
- Iorio, M., et al. Conformational changes on PMMA induced by the presence of TiO 2 nanoparticles and the processing by Solution Blow Spinning. Colloid and Polymer Science. 296 (3), 461-469 (2018).
- Martínez-Sanz, M., et al. Antimicrobial poly (lactic acid)-based nanofibres developed by solution blow spinning. Journal of Nanoscience and Nanotechnology. 15 (1), 616-627 (2015).
- Wang, H., et al. Highly flexible indium tin oxide nanofiber transparent electrodes by blow spinning. ACS Applied Materials and Interfaces. 8 (48), 32661-32666 (2016).
- Greenhalgh, R. D., et al. Hybrid sol-gel inorganic/gelatin porous fibres via solution blow spinning. Journal of Materials Science. 52 (15), 9066-9081 (2017).
- Gonzalez-Abrego, M., et al. Mesoporous titania nanofibers by solution blow spinning. Journal of Sol-Gel Science and Technology. 81 (2), 468-474 (2017).
- Oliveira, J. E., Zucolotto, V., Mattoso, L. H., Medeiros, E. S. Multi-walled carbon nanotubes and poly (lactic acid) nanocomposite fibrous membranes prepared by solution blow spinning. Journal of Nanoscience and Nanotechnology. 12 (3), 2733-2741 (2012).
- Medeiros, E. S., Glenn, G. M., Klamczynski, A. P., Orts, W. J., Mattoso, L. H. Solution blow spinning: A new method to produce micro-and nanofibers from polymer solutions. Journal of Applied Polymer Science. 113 (4), 2322-2330 (2009).
- Vasireddi, R., et al. Solution blow spinning of polymer/nanocomposite micro-/nanofibers with tunable diameters and morphologies using a gas dynamic virtual nozzle. Scientific Reports. 9 (1), 1-10 (2019).
- Tutak, W., et al. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials. 34 (10), 2389-2398 (2013).
- Daristotle, J. L., Behrens, A. M., Sandler, A. D., Kofinas, P. A review of the fundamental principles and applications of solution blow spinning. ACS Applied Materials and Interfaces. 8 (51), 34951-34963 (2016).
- Hofmann, E., et al. Microfluidic nozzle device for ultrafine fiber solution blow spinning with precise diameter control. Lab on a Chip. 18 (15), 2225-2234 (2018).
- Behrens, A. M., et al. In situ deposition of PLGA nanofibers via solution blow spinning. ACS Macro Letters. 3 (3), 249-254 (2014).
- Vural, M., Behrens, A. M., Ayyub, O. B., Ayoub, J. J., Kofinas, P. Sprayable elastic conductors based on block copolymer silver nanoparticle composites. ACS Nano. 9 (1), 336-344 (2015).
- Srinivasan, S., Chhatre, S. S., Mabry, J. M., Cohen, R. E., McKinley, G. H. Solution spraying of poly (methyl methacrylate) blends to fabricate microtextured, superoleophobic surfaces. Polymer. 52 (14), 3209-3218 (2011).
- Flory, P. J. . Principles of polymer chemistry. , (1953).
- Palangetic, L., et al. Dispersity and spinnability: Why highly polydisperse polymer solutions are desirable for electrospinning. Polymer. 55 (19), 4920-4931 (2014).
- Ying, Q., Chu, B. Overlap concentration of macromolecules in solution. Macromolecules. 20 (2), 362-366 (1987).
- Haro-Pérez, C., Andablo-Reyes, E., Díaz-Leyva, P., Arauz-Lara, J. L. Microrheology of viscoelastic fluids containing light-scattering inclusions. Physical Review E. 75 (4), 041505 (2007).
- Thiele, J., et al. Early development drug formulation on a chip: Fabrication of nanoparticles using a microfluidic spray dryer. Lab on a Chip. 11 (14), 2362-2368 (2011).
- Zhao, J., Xiong, W., Yu, N., Yang, X. Continuous jetting of alginate microfiber in atmosphere based on a microfluidic chip. Micromachines. 8 (1), 8 (2017).
- Jun, Y., Kang, E., Chae, S., Lee, S. H. Microfluidic spinning of micro-and nano-scale fibers for tissue engineering. Lab on a Chip. 14 (13), 2145-2160 (2014).
- Weng, B., Xu, F., Salinas, A., Lozano, K. Mass production of carbon nanotube reinforced poly (methyl methacrylate) nonwoven nanofiber mats. Carbon. 75, 217-226 (2014).
- Barton, A. F. Solubility parameters. Chemical Reviews. 75 (6), 731-753 (1975).

