Optimization of Radiochemical Reactions using Droplet Arrays
概要
This method describes the use of a novel high-throughput methodology, based on droplet chemical reactions, for the rapid and economical optimization of radiopharmaceuticals using nanomole amounts of reagents.
Abstract
Current automated radiosynthesizers are designed to produce large clinical batches of radiopharmaceuticals. They are not well suited for reaction optimization or novel radiopharmaceutical development since each data point involves significant reagent consumption, and contamination of the apparatus requires time for radioactive decay before the next use. To address these limitations, a platform for performing arrays of miniature droplet-based reactions in parallel, each confined within a surface-tension trap on a patterned polytetrafluoroethylene-coated silicon “chip”, was developed. These chips enable rapid and convenient studies of reaction parameters including reagent concentrations, reaction solvent, reaction temperature and time. This platform permits the completion of hundreds of reactions in a few days with minimal reagent consumption, instead of taking months using a conventional radiosynthesizer.
Introduction
Positron-emission tomography (PET) radiopharmaceuticals are widely used as research tools to monitor specific in vivo biochemical processes and study diseases, and for the development of new drugs and therapies. Moreover, PET is a critical tool for diagnosing or staging disease and monitoring a patient's response to therapy1,2,3. Due to the short half-life of PET radioisotopes (e.g., 110 min for fluorine-18-labeled radiopharmaceuticals) and radiation hazard, these compounds are prepared using specialized automated systems operating behind radiation shielding and must be prepared just before use.
Current systems used to synthesize radiopharmaceuticals are designed to produce large batches that are divided up into many individual doses to share the production cost. While current systems are suitable for the production of widely used radiotracers like [18F]FDG (because multiple patient scans and research experiments can be scheduled in a single day), these systems can be wasteful for the production of novel radiotracers during early-stage development, or less commonly used radiotracers. Volumes that conventional systems use are typically in the 1-5 mL range, and the reactions require precursor amounts in the 1-10 mg range. Furthermore, using conventional radiosynthesizers is generally cumbersome during optimization studies since the apparatus becomes contaminated after use and the user must wait for radioactivity to decay before performing the next experiment. Aside from equipment cost, the cost of the radioisotope and reagents can, therefore, become very substantial for studies requiring production of multiple batches. This can occur, for example, during the optimization of synthesis protocols for novel radiotracers to achieve sufficient yield and reliability for initial in vivo imaging studies.
Microfluidic technologies have been increasingly used in radiochemistry to capitalize on several advantages over conventional systems4,5,6. Microfluidic platforms, including those based on 1-10 µL reaction volumes7,8,9, have shown a significant reduction of reagent volumes and consumption of expensive precursors, as well as short reaction times. These reductions lead to lower costs, faster heating and evaporation steps, shorter and more straightforward downstream purification, an overall "greener" chemistry process10, and higher molar activity of the produced radiotracers11. These improvements make it more practical to perform more extensive optimization studies by lowering the reagent cost of each synthesis. Further benefits can be achieved by performing multiple experiments from a single batch of radioisotope in a single day. For example, microfluidic flow chemistry radiosynthesizers operating in "discovery mode" can sequentially perform dozens of reactions, each using only 10s of µL reaction volume12.
Inspired by these advantages, a multi-reaction droplet array chip in which microvolume reactions are confined to an array of surface-tension traps on a silicon surface, created using a patterned Teflon coating, was developed. These chips enable multiple reactions at the 1-20 µL scale to be performed simultaneously, opening the possibility to explore 10s of different reaction conditions per day, each with multiple replicates. In this paper, the utility of this new high-throughput approach for performing rapid and low-cost radiochemistry optimizations is demonstrated. Using multi-reaction droplet chips allows for convenient exploration of the impact of reagent concentrations and reaction solvent, and the use of multiple chips could enable the study of reaction temperature and time, all while consuming very low amounts of precursor.
Protocol
CAUTION: This protocol involves the handling of radioactive materials. Experiments should not be undertaken without the necessary training and personal protective equipment and approval from the radiation safety office at your organization. Experiments should be performed behind radiation shielding, preferably in a ventilated hot cell
1. Fabrication of multi-reaction chips
NOTE: Batches of multi-reaction microdroplet chips are fabricated from 4" silicon wafers using standard photolithography techniques, as previously decribed10 (Figure 1). This procedure will produce 7 chips each with 4 x 4 array of reaction sites.
- Place silicon wafer on the spin-coater chuck, ensuring that it is centered. Deposit 3 mL of polytetrafluoroethylene solution at the center of the wafer with a transfer pipette and coat wafer at a 1000 rpm for 30 s (500 rpm/s ramp).
- To solidify the coating, place the wafer on a 160 °C hotplate for 10 min and then transfer to a 245 °C hotplate for 10 min.
- Anneal the coating in a high-temperature oven at 340 °C for 3.5 h under nitrogen atmosphere, followed by cooling to 70 °C at a 10 °C/min ramp.
- Place the silicon wafer on the spin-coater chuck, ensuring that it is centered. Pour 2 mL of positive photoresist at the center of the wafer using a transfer pipette, and then perform coating at 3000 rpm for 30 s (1000 rpm/s ramp).
- Solidify the photoresist by performing a soft bake of the wafer on a 115 °C hotplate for 3 min.
- Install the wafer and photomask in a mask aligner and perform a 14 s exposure at 12 mW/cm2 lamp intensity and 356 nm wavelength in hard contact mode. This step uses a transparency mask containing the negative final polytetrafluoroethylene pattern, i.e., a 4" diameter pattern of 4 copies of the 16-reaction chip, with reaction sites transparent and all other regions in opaque color.
- Submerge the wafer using 20 mL of photoresist developer solution in a glass container for 3 min with slight agitation to develop the exposed pattern.
- Rinse away the developing solution by submerging the wafer in a glass container with 20 mL of DI water for 3 min with slight agitation. Dry the wafer with a nitrogen gun.
- Remove the exposed polytetrafluoroethylene regions via reactive-ion etching (RIE) with oxygen plasma under the following conditions: 30 s exposure, 100 mTorr pressure, 200 W power, and 50 sccm oxygen flow.
- Dice the wafer into individual chips (7 total per wafer) using a silicon wafer cutter.
- Submerge each chip in acetone for 1 min to remove the photoresist, then isopropanol for 1 min. Finally, dry each chip with a nitrogen gun.
- Place dry chips in a glass container and cover with aluminum foil for storage until use.
2. Planning of the optimization study
NOTE: In this protocol, synthesis of the radiopharmaceutical [18F]fallypride is used as an example to illustrate high-throughput optimization (Figure 2). With a single chip, 16 simultaneous reactions can be performed, for example, with varied precursor concentration (8 different concentrations, n=2 replicates each). The conditions are mapped to reaction sites in Figure 3A. Adjustments can be made to this protocol to optimize other reaction parameters (e.g. reaction solvent, reaction volume, amount of TBAHCO3, etc.) or other radiopharmaceuticals.
- Select the reaction parameter(s) to be varied, the specific values to be used, and the number of replicates.
- Compute the number of chips needed to perform the experiment.
- For each chip, prepare a map of which reaction conditions will be used at each reaction site to assist with reagent preparation and performing the droplet reactions.
3. Preparation of reagents and materials for optimizing the radiosynthesis of [18F]fallypride
NOTE: The droplet-based radiosynthesis of [18F]fallypride (Figure 2) begins with the addition of [18F]fluoride and phase transfer catalyst (TBAHCO3) to the reaction site, followed by heating to evaporate water and leave a dried residue. Next, a droplet of precursor (tosyl-fallypride) in reaction solvent (thexyl alcohol and acetonitrile) is added and heated to perform the radiofluorination reaction. Finally, the crude product is collected from the chip for analysis. The reagent preparation and synthesis procedures should be adapted if performing optimization of a different tracer.
- Prepare a stock solution of the reaction solvent, consisting of thexyl alcohol and acetonitrile in a 1:1 by volume mixture. Ensure that the volume is enough to create the planned dilution series. In this example optimization, ~30 µL is sufficient.
- Prepare a 30 µL stock solution of precursor (tosyl-fallypride) in the reaction solvent with the maximum concentration to be explored (77 mM). Ensure that the volume is enough to perform the planned experiment. In this example optimization, ~30 µL is sufficient.
- From the precursor stock solution and reaction solvent, perform 2x serial dilutions to prepare the different concentrations of the precursor solution. Ensure that the volume of each dilution is enough to perform the desired number of replicates for each condition. In this example optimization, ~15 µL of each concentration is sufficient.
- Prepare microcentrifuge tubes to collect each crude reaction product using a permanent marker to label each tube with a unique number. Ensure that the total number of microcentrifuge tubes matches the number of conditions multiplied by the number of replicates (8 x 2 = 16).
- Prepare a stock of collection solution (10 mL) comprising 9:1 methanol:DI water (v/v). Aliquot 50 µL into each of 16 additional labeled microcentrifuge tubes (one per reaction site on the chip).
- Prepare a [18F]fluoride stock solution in a 500 µL microcentrifuge tube by mixing [18F]fluoride/[18O]H2O (~260 MBq [7 mCi]) with 75 mM TBAHCO3 solution (56 µL) and diluting with DI water up to 140 µL. 8 µL of this solution will be loaded to each reaction site (containing ~15 MBq [0.40 mCi] of activity, and 240 nmol of TBAHCO3).
4. Parallel synthesis of [18F]fallypride with different precursor concentrations
NOTE: The chip is operated atop a heating platform (constructed as previously described13) consisting of a 25 mm x 25 mm ceramic heater, controlled using an on-off temperature controller using the internal thermocouple signal for feedback. Heater surface temperatures were calibrated using thermal imaging. If such a platform is not available, a pair of hot plates can be used (one at 105 °C and one at 110 °C).
- Load [18F]fluoride stock solution (with phase transfer catalyst).
- Using a micropipette, load an 8 µL droplet of [18F]fluoride stock solution on the first reaction spot of a multi-reaction chip. Measure the activity of the chip by placing it in a dose calibrator and record the time at which measurement is conducted.
- Remove the chip from dose calibrator and then load an 8 µL droplet of [18F]fluoride stock solution on the second reaction spot. Measure the activity on the chip by placing it once again in the dose calibrator and record the time at which measurement is conducted.
- Repeat for all other reaction sites on the chip.
- Calculate the activity loaded per reaction spot by taking the activity measurement after loading the radioisotope and subtracting the previous measurement (decay-corrected) before that site was loaded.
- Align the multi-reaction chip on the heater.
- Add a thin layer of thermal paste on top of the ceramic heater.
- Carefully place the chip on top of the heater using tweezers to avoid the spill of the droplets, aligning the reference corner of the chip with the reference corner of the heater (as shown in Figure 3B). The chip will overhang the heater by a small amount.
- Dry the [18F]fluoride and phase transfer catalyst.
- Heat the chip for 1 min by setting the heater to 105 °C in the control program to evaporate the droplets to dryness leaving a dried residue of [18F]fluoride and TBHACO3. After 1 min, cool the chip by turning off the heater and turning on the cooling fan with the control program.
- Add the precursor solution.
- Using a micropipette, add a 6 µL solution of fallypride precursor on top of the dried residue on the first reaction site.
- Repeat for all other reaction sites on the chip. Use the optimization plan to determine which concentration of the dilution series is used for each reaction site.
- Perform fluorination reaction.
- Heat each chip to 110 °C for 7 min using the control program to perform radiofluorination reaction. Afterwards, cool the chip by turning off the heater and turning on cooling fan with the control program.
- Collect the crude products from the reaction sites.
- Collect the crude product at the first reaction site by adding 10 µL of collection solution from the designated microcentrifuge tube via micropipette. After waiting for 5 s, use the micropipette (with the same tip installed) to aspirate the diluted crude product and transfer to its corresponding labeled collection microcentrifuge tube.
- Repeat this process a total of 4 times using the same pipette tip for all operations.
- Repeat the collection process for all other reaction sites on the chip.
5. Synthesis analysis to determine reaction performance and optimal conditions
- Determine the "collection efficiency" for the first reaction on the chip.
- Place the microcentrifuge tube with the collected crude product of the first reaction spot in the dose calibrator to measure the activity. Record the measurement and time of the measurement.
- Calculate the collection efficiency by dividing the activity of the collected crude product by the starting activity measured for the same reaction site (decay-correcting the activity values to the same timepoint).
- Repeat for all other reaction sites on the chip.
- Analyze the composition (fluorination efficiency) of each collected crude product.
NOTE: To make practical the analysis of all samples in a short time, fluorination efficiency is analyzed using a previously described high-throughput radio-thin layer chromatography (radio-TLC) approach14. This technique allows up to eight samples to be processed in parallel by spotting then side by side (5 mm pitch, 0.5 µL per spot) on a single TLC plate, then developing together, and performing readout together using Cerenkov imaging14, 15. For the example optimization with 16 parallel reactions, 2 TLC plates are needed. Another option is to use radio- high-performance liquid chromatography (radio-HPLC) for analysis, though the time for separation, cleaning, and equilibration may limit the number of samples that can be analyzed.- For each TLC plate (50 mm x 60 mm), with a pencil, draw a line at 15 mm away from one 50 mm edge (bottom), and another line 50 mm away from the same edge. The first line is the origin line; the second is the solvent front line. Draw 8 small "X"s along the origin line at 5 mm spacing to define the sample spotting position for each of 8 "lanes".
- Using a micropipette, transfer 0.5 µL of the first crude product onto the TLC plate at the "X" for the first lane. Repeat for additional crude products (up to 8 per TLC plate). Wait for the crude product spots to dry on the TLC plate.
- For each TLC plate, develop using a mobile phase of 60% MeCN in 25 mM NH4HCO2 with 1% TEA (v/v) until the solvent front reaches the solvent front line. Wait for the solvent on the TLC plate to dry and then cover with a glass microscope slide (76.2 mm x 50.8 mm, 1 mm thick).
- Obtain a radioactivity image of each TLC plate by placing the plate in a Cerenkov imaging system for a 5 min exposure. Perform standard image corrections (dark current subtraction, flat field correction, median filtering, and background subtraction).
- Use region of interest (ROI) analysis for the first lane of the first TLC plate. Draw regions around each band visible in the lane. The software will compute the fraction of integrated intensity of each region (band) compared to the total integrated intensity of all regions (bands).
- With this mobile phase, the following bands are expected at the indicated retention factors: Rf = 0.0: Unreacted [18F]fluoride; Rf = 0.9: [18F]fallypride; Rf = 0.94: Side product. Determine the fluorination efficiency as the fraction of activity in the [18F]fallypride band.
- Repeat this analysis for all other lanes on all TLC plates.
NOTE: If a Cerenkov imaging chamber is not available, a small animal (preclinical) in vivo optical imaging system can be used to image the TLC plates. Alternatively, a 2-dimensional TLC scanner can be used. Alternatively, if only a 1-dimensional TLC scanner is available, the TLC plates can be analyzed by cutting into strips with scissors (1 per lane), and scanning each strip individually.
- Determine the crude radiochemical yield (crude RCY) for each reaction site.
- Determine the crude RCY for the first crude product by multiplying the collection efficiency by the fluorination efficiency.
- Repeat for all other reaction sites.
- Analyze the results
- Aggregate values for any replicate experiments into an average and standard deviation.
- Plot the collection efficiency, fluorination efficiency, and crude RCY as a function of the parameter that was varied (precursor concentration in this example).
- Select the optimal conditions based on the desired criteria. Typically, this is the maximum crude RCY. Additionally, the point is often chosen in a region where the slope of the graph is relatively flat, indicating it is insensitive to small changes in the parameter, providing a more robust protocol.
Representative Results
A representative experiment was performed to illustrate this method. Using 16 reactions, optimization studies of the radiopharmaceutical [18F]fallypride were performed by varying precursor concentration (77, 39, 19, 9.6, 4.8, 2.4, 1.2, and 0.6 mM) in thexyl alcohol:MeCN (1:1, v/v) as the reaction solvent. Reactions were performed at 110 °C for 7 min. Collection efficiency, sample composition (i.e., proportions of [18F]fallypride product, unreacted [18F]fluoride, and side product) are tabulated in Table 1 and are summarized graphically in Figure 4.
The study showed that the fluorination efficiency (proportion of [18F]fallypride) increases with increasing precursor concentration, and that the remaining unreacted [18F]fluoride varied inversely (Figure 4A). There was a small amount of a radioactive side product at low precursor concentrations, but the proportion decreased to near zero at the higher precursor concentrations (Figure 4A). The collection efficiency was nearly quantitative for most conditions, though it dropped slightly at low precursor concentrations.
From these results, the highest RCY can be achieved with ~230 nmol of precursor (i.e., 39 mM concentration in a 6 µL droplet). At this condition, the fluorination efficiency was 96.0 ± 0.5% (n=2) and the crude RCY was 87.0 ± 2.7 (n=2), and there was no observed radioactive side product formation. While the use of 77 mM precursor showed similar results, in general it is desirable to use a lower amount of precursor to reduce cost and simplify downstream purification steps.
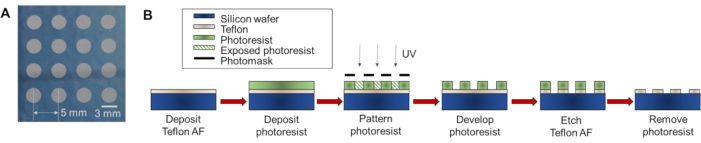
Figure 1: Fabrication of multi-reaction microdroplet chips via photolithography. (A) Photograph of multi-reaction microdroplet chip with 4 x 4 array of reaction sites. The chip consists of polytetrafluoroethylene -coated silicon with circular regions of polytetrafluoroethylene etched away to create the hydrophilic reaction sites. (B) Schematic of the fabrication procedure. A silicon wafer is spin-coated with Teflon solution and baked to solidify the coating. Next, the photoresist is spin-coated and patterned via photolithography to produce an etch mask. The photoresist is developed with a photoresist developing solution. The exposed Teflon is then removed via dry etching with oxygen plasma. The wafer is diced into individual chips, and the photoresist is stripped. Please click here to view a larger version of this figure.
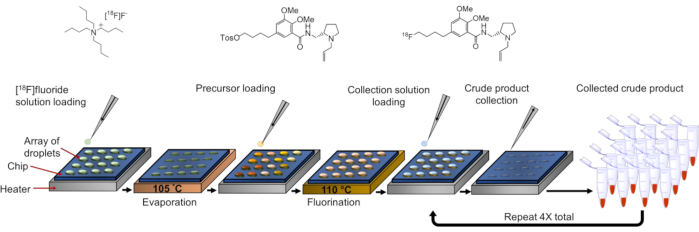
Figure 2: Procedure for parallel reactions. Experimental procedure for performing 16 parallel syntheses of the radiopharmaceutical [18F]fallypride on a multi-reaction chip. In this example, the precursor concentration is varied for each reaction. Please click here to view a larger version of this figure.
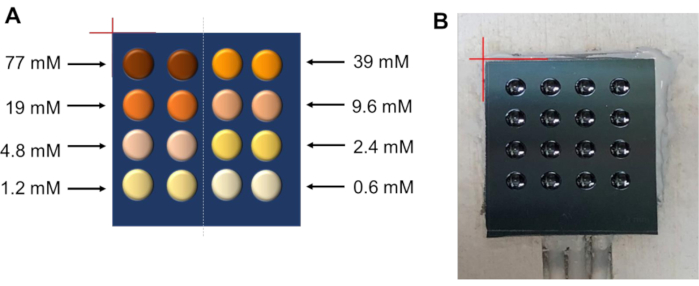
Figure 3: Map of conditions at reaction sites. (A) Experimental design to explore the influence of precursor concentration on the radiofluorination of tosyl fallypride using a single 16-reaction chip (top view). Eight different concentrations were explored, each with n=2 replicates. Other reaction conditions were held constant (temperature: 110 °C; time: 7 min; solvent: thexyl alcohol:MeCN; the amount of TBAHCO3: 240 nmol). Each reaction was performed with ~14 MBq of activity. (B) Photograph of a 16-reaction chip installed on the heater platform during the experiment. Red lines represent the reference corner of the chip used for alignment with the reference corner of the heater. Please click here to view a larger version of this figure.
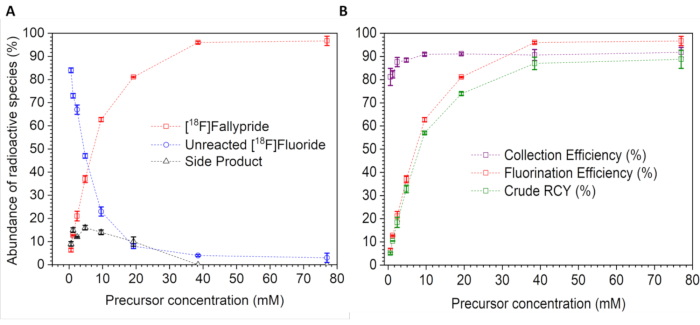
Figure 4: Influence of precursor concentration on the microdroplet synthesis of [18F]fallypride. (A) Proportion of radioactive species present in the collected crude reaction product, i.e. [18F]fallypride, side product, or unreacted [18F]fluoride. (B) Synthesis performance. Collection efficiency, fluorination efficiency, and crude RCY are plotted as a function of precursor concentration. In both graphs, data points represent the average of n=2 replicates, and error bars represent the standard deviation. Please click here to view a larger version of this figure.
| Precursor concertation (mM) | Collection efficiency (%) | Fluorination efficiency (%) | Crude RCY (%) | Unreacted [18F]fluoride (%) | Side product (%) |
| 77 | 91.8 ± 2.1 | 96.7 ± 2.0 | 88.8 ± 3.9 | 3.3 ± 2.0 | 0.0 ± 0.0 |
| 39 | 90.6 ± 2.4 | 96.0 ± 0.5 | 87.0 ± 2.7 | 4.0 ± 0.5 | 0.0 ± 0.0 |
| 19 | 91.1 ± 0.5 | 81.1 ± 0.3 | 73.9 ± 0.7 | 8.4 ± 1.2 | 10.5 ± 2.0 |
| 9.6 | 90.9 ± 0.6 | 62.7 ± 0.9 | 57.0 ± 0.5 | 23.3 ± 2.1 | 14.0 ± 0.9 |
| 4.8 | 88.4 ± 0.8 | 37.0 ± 1.5 | 32.8 ± 1.6 | 47.3 ± 0.8 | 15.7 ± 1.0 |
| 2.4 | 87.6 ± 2.0 | 21.0 ± 2.1 | 18.4 ± 2.2 | 67.4 ± 2.1 | 11.6 ± 1.0 |
| 1.2 | 82.3 ± 1.6 | 12.7 ± 0.3 | 10.4 ± 0.1 | 72.8 ± 0.7 | 14.5 ± 1.0 |
| 0.6 | 81.2 ± 3.7 | 6.3 ± 0.8 | 5.1 ± 0.5 | 84.3 ± 0.2 | 9.4 ± 1.0 |
Table 1: Data obtained from study of precursor concentration. All values are averages ± standard deviations computed from n=2 replicates.
Discussion
Due to limitations of conventional radiochemistry systems that allow only one or a small number of reactions per day and consume a significant quantity of reagents per data point, only a tiny portion of the overall reaction parameter space can be explored in practice, and many times results are reported with no repeats (n=1). Compared to conventional systems, this multi-reaction droplet radiosynthesis platform makes it practical to accomplish more comprehensive and rigorous studies of radiosynthesis conditions while consuming very little time and amount of precursor, potentially enabling new insights on parameters that impact product yield and side-product formation. The information can be used to choose the conditions that result in the highest product yield or the most robust synthesis. The low precursor consumption may be especially useful in the early development of novel radiotracers when only a small amount of precursor may be available or when the precursor is expensive. While the open nature of the chips contributes to rapid synthesis time and ease of access via pipette, it can lead to substantial losses of volatile molecules and may not be practical when optimizing the synthesis of radiopharmaceuticals that have volatile precursors, intermediates, or products.
Due to the hazard of radiation exposure, it should be reiterated that these experiments should be performed only with suitable training and approvals and should be conducted behind radiation shielding, preferably in a ventilated hot cell. Due to the short half-life of the radioisotopes, it is important to perform the experiments quickly and efficiently. Pipetting reagents to the chip and collecting products from the chip should be practiced under non-radioactive conditions to become familiar with the reduced access and visibility in a hot cell. Similarly, installing and removing the chip, and making measurements of the chip with the dose calibrator should also be practiced. In addition, it is critical to be organized, with a detailed experiment map (i.e., specific reaction conditions at each site on the chip). It is also helpful to prepare in advance a table of results to be filled in as measurements are made. To ensure reproducibility, especially with the possibility of human error, multiple replicates of each set of conditions should be performed. It is important to be especially careful during the step of collecting the crude samples from the chip to avoid spilling liquid outside the reaction site and causing cross-contamination with adjacent reaction sites. If any errors are noticed, it is important to flag these reaction sites so the data can be excluded from the eventual analysis.
In this example study, the amount precursor consumed for 16 data points was 1.1 mg (~70 µg each), compared to 4 mg per data point using a conventional radiosynthesizer. Furthermore, all 16 reactions were completed in 25 min all in a single experiment. In comparison, the synthesis of crude [18F]fallypride on a conventional radiosynthesizer requires ~15-20 min per reaction16, 17.
This representative experiment demonstrated the utility of a multi-reaction microdroplet chip with 16 reactions to optimize conditions for the radiosynthesis of the radiopharmaceutical [18F]fallypride by exploring 8 different precursor concentrations (n=2 replicates for each condition) in a fast and economical manner. Other variables that can be conveniently optimized using a multi-reaction chip include the amount of radioactivity, type of phase transfer catalyst, amount of phase transfer catalyst, evaporation/drying conditions (e.g., number of azeotropic drying steps), reaction solvent, etc. By using multiple multi-reaction chips, it also is possible to explore the influence of reaction temperature and reaction time, in addition to conditions such as evaporation/drying temperature and time. Such studies would need to be performed sequentially using the single heater or could be parallelized by operating multiple heaters at the same time.
The underlying droplet synthesis method has been shown to be compatible with a wide range of 18F-labeled radiopharmaceuticals, such as [18F]fallypride10, [18F]FET18, [18F]FDOPA19, [18F]FBB20 and it can be used for the optimization of the majority of other 18F-labeled compounds and compounds labeled with other isotopes. Moreover, the resulting optimized droplet-based reactions intrinsically leverage the advantages of microvolume radiochemistry, including reduced precursor consumption, faster process times, and compact instrumentation, and can offer these same advantages for routine production of large batches. Larger batches simply require scaling up the amount of activity initially loaded at the start of the reaction. To prepare a tracer suitable for use in in vitro or in vivo assays, the crude product must be purified (e.g., using analytical-scale HPLC) and formulated (e.g. via evaporative or solid-phase solvent exchange21) Alternatively, it may be possible to adapt the optimal conditions from droplet-scale to a conventional vial-based radiosynthesizer. Investigation of this possibility is ongoing.
開示
The authors have nothing to disclose.
Acknowledgements
We thank the UCLA Biomedical Cyclotron Facility and Dr. Roger Slavik and Dr. Giuseppe Carlucci for generously providing [18F]fluoride for these studies and the UCLA NanoLab for support with equipment for chip fabrication.
Materials
| 2,3-dimethyl-2-butanol (thexyl alcohol) | Sigma-Aldrich | 594-60-5 | 98% |
| Acetone | KMG Chemicals | Cleanroom LP grade | |
| Ammonium formate (NH4HCO2) | Sigma-Aldrich | 540-69-2 | 97% |
| Anhydrous acetonitrile (MeCN) | Sigma-Aldrich | 75-05-8 | 99.80% |
| Ceramic heater | Watlow | Utramic CER-1-01-0093 | 25 mm x 25 mm |
| Cerenkov imaging chamber | Custom built | Other instruments can be used for TLC plate readout including: small animal in vivo optical imaging system, 2D radio-TLC scanner, 1D radio-TLC scanner | |
| DI water | Sigma-Aldrich | 7732-18-5 | |
| Disposable transfer pipets, 3 mL | Falcon | 13-680-50 | |
| Dose calibrator | Capintec, Inc. | CRC-25 PET | |
| Fallypride | ABX Advanced Biochemical Compounds | 1560.0010.000 | Fallypride reference standard, >95% |
| [18F]fluoride in [18O]H2O | UCLA Ahmanson Biomedical Cyclotron Facility | Due to short half-life this must be obtained from local radiochemistry lab or commercial radiopharmacy | |
| Glass cover plates (76.2 mm x 50.8 mm x 1 mm thick) | C&A Scientific | 6101 | |
| Headway spin coater | Headway Research, Inc. | PWM50-PS-R790 Sipinner system | PWM50-control box, PS-motor, R790-bowl |
| High temperature oven | Carbolite | HTCR 6 28 | |
| Hot plate | Thermo Scientific | Super-Nuova HP133425 | |
| Isopropanol (IPA) | KMG Chemicals | Cleanroom LP grade | |
| Mask aligner | Karl Suss | MA/BA6 | |
| Methanol (MeOH) | Sigma-Aldrich | 67-56-1 | ≥99.9% |
| Microcentrifuge tube | Eppendorf | 0030 123.301 | 500 µL, colorless, polypropylene |
| Micropipette (0.5-10 µL) | Labnet | BioPette P3940-10 | |
| Micropipette (100-1000 µL) | Labnet | BioPette P3940-1000 | |
| Micropipette (10-100 µL) | Labnet | BioPette P3940-100 | |
| Micropipette tips (0.1-10 µL) | USA Scientific Inc Tips | 11113810 | |
| Micropipette tips (2-200 µL) | BrandTech | 13-889-143 | |
| Micropipette tips (50-1000 µL) | BrandTech | 13-889-145 | |
| Photoresist developer solution | MicroChem | MEGAPOSIT MF-26A | |
| Positive photoresist | MicroChem | MEGAPOSIT 220-7.0 | |
| Reactive-ion etcher (RIE) | Oxford Instruments | Plasma Lab 80 Plus | |
| Silicon wafer cutter | Euro Tool | CSCB-431.00 | |
| Silicon wafer; 4" diameter | Silicon Valley Microelectronics Inc. | 0017227-048 | P type, boron doped, thickness 525 ± 25 µm |
| Teflon AF 2400 | Chemours | D14896765 | 1% solids |
| Tetrabutylammonium bicarbonate (TBAHCO3) | ABX Advanced Biochemical Compounds | 808 | Aqueous solution stabilized with ethanol, 0.075 M |
| Themal conducting paste | OMEGA | OT-201-2 | |
| TLC plates | Merck KGaA | 1.05554.0001 | Silica gel 60 F254, 50 mm x 60 mm, aluminum back |
| Tosyl-fallypride | ABX Advanced Biochemical Compounds | 1550.004.000 | Fallypride precursor, >90% |
| Trimethylamine (TEA) | Sigma-Aldrich | 75-50-3 | ≥ 99% |
| Tweezers | Cole-Parmer | UX-07387-08 | Stainless steel, fine tip |
参考文献
- Matthews, P. M., Rabiner, E. A., Passchier, J., Gunn, R. N. Positron emission tomography molecular imaging for drug development. British Journal of Clinical Pharmacology. 73 (2), 175-186 (2012).
- Piel, M., Vernaleken, I., Rösch, F. Positron emission tomography in CNS drug discovery and drug monitoring. Journal of Medicinal Chemistry. 57 (22), 9232-9258 (2014).
- Cherry, S. R., Sorenson, J. A., Phelps, M. E. . Physics in Nuclear Medicine. , (2012).
- Knapp, K. -. A., Nickels, M. L., Manning, H. C. The current role of microfluidics in radiofluorination chemistry. Molecular Imaging and Biology. 22 (3), 463-475 (2020).
- Rensch, C., et al. Microfluidics: A groundbreaking technology for PET tracer production. Molecules. 18 (7), 7930-7956 (2013).
- Pascali, G., Watts, P., Salvadori, P. A. Microfluidics in radiopharmaceutical chemistry. Nuclear Medicine and Biology. 40 (6), 776-787 (2013).
- Keng, P. Y., van Dam, R. M. Digital microfluidics: A new paradigm for radiochemistry. Molecular Imaging. 14, 579-594 (2015).
- Wang, J., Chao, P. H., Janet, S., van Dam, R. M. Performing multi-step chemical reactions in microliter-sized droplets by leveraging a simple passive transport mechanism. Lab on a Chip. 17 (24), 4342-4355 (2017).
- Wang, J., Chao, P. H., van Dam, R. M. Ultra-compact, automated microdroplet radiosynthesizer. Lab on a Chip. (19), 2415-2424 (2019).
- Rios, A., Wang, J., Chao, P. H., van Dam, R. M. A novel multi-reaction microdroplet platform for rapid radiochemistry optimization. RSC Advances. 9 (35), 20370-20374 (2019).
- Sergeev, M., et al. Performing radiosynthesis in microvolumes to maximize molar activity of tracers for positron emission tomography. Communications Chemistry. 1 (1), 10 (2018).
- Pascali, G., et al. Optimization of nucleophilic 18F radiofluorinations using a microfluidic reaction approach. Nature Protocols. 9 (9), 2017-2029 (2014).
- Lisova, K., et al. Microscale radiosynthesis, preclinical imaging and dosimetry study of [18F]AMBF3-TATE: A potential PET tracer for clinical imaging of somatostatin receptors. Nuclear Medicine and Biology. 61, 36-44 (2018).
- Wang, J., et al. High-throughput radio-TLC analysis. Nuclear Medicine and Biology. 82-83, 41-48 (2020).
- Dooraghi, A. A., et al. Optimization of microfluidic PET tracer synthesis with Cerenkov imaging. Analyst. 138 (19), 5654-5664 (2013).
- Collins, J., et al. Production of diverse PET probes with limited resources: 24 18F-labeled compounds prepared with a single radiosynthesizer. Proceedings of the National Academy of Sciences. 114 (43), 11309-11314 (2017).
- Lazari, M., et al. Fully automated production of diverse 18F-labeled PET tracers on the ELIXYS multireactor radiosynthesizer without hardware modification. Journal of Nuclear Medicine Technology. 42 (3), 203-210 (2014).
- Lisova, K., et al. Rapid, efficient, and economical synthesis of PET tracers in a droplet microreactor: application to O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET). EJNMMI Radiopharmacy and Chemistry. 5 (1), 1 (2019).
- Wang, J., Holloway, T., Lisova, K., van Dam, R. M. Green and efficient synthesis of the radiopharmaceutical [18F]FDOPA using a microdroplet reactor. Reaction Chemistry & Engineering. 5 (2), 320-329 (2020).
- Lisova, K., Wang, J., Rios, A., van Dam, R. M. Adaptation and optimization of [F-18] Florbetaben ([F-18] FBB) radiosynthesis to a microdroplet reactor. Journal of Labelled Compounds and Radiopharmaceuticals. 62, 353-354 (2019).
- Wang, J., Chao, P. H., Slavik, R., van Dam, R. M. Multi-GBq production of the radiotracer [18F]fallypride in a droplet microreactor. RSC Advances. 10 (13), 7828-7838 (2020).

