Imaging Dpp Release from a Drosophila Wing Disc
概要
The timing of exposure to ligands may impact their developmental consequences. Here we show how to image release of a Drosophila bone morphogenetic protein (BMP) called Dpp from cells of the wing disc.
Abstract
The transforming Growth Factor-beta (TGF-β) superfamily is essential for early embryonic patterning and development of adult structures in multicellular organisms. The TGF-β superfamily includes TGF-β, bone morphogenetic protein (BMPs), Activins, Growth and Differentiation Factors, and Nodals. It has long been known that the amount of ligand exposed to cells is important for its effects. It was thought that long-range concentration gradients set up embryonic pattern. However, recently it has become clear that the timing of exposure to these ligands is also important for their downstream transcriptional consequences. A TGF-β superfamily ligand cannot have a developmental consequence until it is released from the cell in which it was produced. Until recently, it was difficult to determine when these ligands were released from cells. Here we show how to measure the release of a Drosophila BMP called Decapentaplegic (Dpp) from the cells of the wing primordium or wing disc. This method could be modified for other systems or signaling ligands.
Introduction
Bone Morphogenetic Proteins (BMPs) are essential for early embryogenesis and patterning of adult structures. BMPs are produced and secreted to affect transcription of target genes needed for growth and cell differentiation in responding cells. Decapentaplegic (Dpp) is a Drosophila homolog of BMP4 that is important for development of embryonic and adult structures like the wing1,2,3,4. Several groups have focused on the role of Dpp in patterning adult fly wings because 1) the wings are comprised of two transparent epithelia sheets with a consistent venation pattern that can be easily assessed; 2) the wing discs are also reasonably flat, can be cultured outside of the larva, and are simple to image and quantify differences in pattern; and 3) the wing pattern development is sensitive to Dpp such that small perturbations in the pathway will impact wing venation pattern.
Dpp is produced in cells located in the anterior/posterior boundary of the wing disc5,6,7,8. Dpp binds to a complex of type 1 and type 2 serine/threonine kinase receptors9,10. Upon Dpp binding, the type 2 receptor phosphorylates the type 1 receptor which then phosphorylates Mothers against Dpp (Mad), a Smad 1/5/8 homolog. Phosphorylated SMAD recruits an additional co-Smad (Medea), which enables it enter to the nucleus where it regulates target genes, leading to downstream effects such as proliferation or differentiation4,11.
Recently, the Bates Lab has shown that the improper release of Dpp within the wing disc can lead to a decrease in Mad phosphorylation, reduction in target gene expression, and wing patterning defects12,13. Several ion channels impact development of the Drosophila wing and associated structures14,15. These ion channels could also be involved in Dpp release. In determining the mechanism of morphogen release, it is important that there is a method to visualize release events.
Drs. Aurelio Teleman and Stephen Cohen created a Dpp-GFP fusion protein which is able to rescue loss of Dpp, meaning that it is biologically active and is released in a biologically relevant manner16. Here, we describe how we visualize Dpp release events using this Dpp-GFP. This fusion protein is particularly useful because GFP is pH sensitive such that when it is in acidic vesicles, the fluorescence is quenched17. Therefore, when a protein tagged with GFP is released from a vesicle into the more neutral extracellular environment, GFP fluorescence intensity increases17. We took advantage of the pH sensitivity of GFP to determine if Dpp-GFP resides in acidic vesicles. We imaged wing discs expressing Dpp-GFP before and after the addition of ammonium chloride, which neutralizes intracellular compartments vesicles18. We found a significant increase in fluorescence of puncta after the addition of ammonium chloride, suggesting that intracellular Dpp-GFP is quenched before the addition of ammonium chloride18. We conclude that intracellular Dpp-GFP resides in acidic membrane-bound compartments, such as vesicles, and is unquenched upon addition of ammonium chloride to neutralize the pH of intracellular compartments18. This makes live imaging of Dpp-GFP a useful technique to visualize the dynamics of Dpp in the Drosophila wing disc as it is released from acidic compartments into the extracellular environment.
Here, we describe the method we use to visualize Dpp release events using Dpp-GFP. Dpp-GFP can be expressed in its native pattern in Drosophila wing discs using the UAS-GAL4 system19. This is the method that was used to determine that Irk channels impact Dpp release18. We validated the method by live-imaging z-stacks. We do not see Dpp-GFP puncta moving within the plane of focus in time series if we acquired in one plane of focus. We also do not see movement of Dpp-GFP puncta if we imaged in a z-stack. We conclude that the Dpp-GFP puncta seen using this method are release events rather than movement of vesicles intracellularly. This method of live-imaging of Dpp-GFP could potentially be used to test other putative modifiers of Dpp release for their impact on Dpp dynamics or could be modified to look at the dynamics of other ligands
Protocol
1. Collecting Eggs to Generate Larvae for Dissection
- Cross 30-40 virgin female Dpp-GAL4/TM6 Tb Hu flies to 10-15 male Sp/CyO-GFP; UAS-Dpp-GFP/TM6 Tb Hu.
NOTE: Any two genotypes containing Dpp-GAL4 and UAS-Dpp-GFP may be used as long as the balancers have larval markers allowing for selection of the appropriate progeny during the larval stage. - To collect eggs, flip the crossed flies into a fresh vial of food and allow them to lay for 3–4 h before removing them from the vial. To encourage egg laying, a small amount of yeast paste made of granulated yeast mixed with a small amount of water can be added to the top of the food before the flies are introduced to the vial. The same set of crossed flies can be kept and used for up to 7 days of egg collections.
NOTE: Any standard Drosophila cornmeal food recipe should be acceptable. Used here is the food recipe described by Hazegh and Reis20. - Keep the egg collection vials at 25 °C for approximately 144 h until larvae are at the third instar (wandering) stage.
- To select the appropriate larvae for dissection (those expressing both Dpp-GAL4 and UAS-Dpp-GFP) first choose larvae that are non-Tb. These larvae will be normal length rather than short and fat.
- Among the non-Tb larvae there will be larvae expressing CyO-GFP and larvae expressing Dpp-GFP. To select the Dpp-GFP expressing larvae, view them under a fluorescence stereomicroscope. While both Dpp-GFP and CyO-GFP expressing larve will have some GFP fluorescence, the Dpp-GFP expressing larvae can be distinguished in that the GFP is restricted to the wing discs and the fluorescence is much less bright than the CyO-GFP expressing larvae. Select these less bright larvae for dissection.
2. Preparing Solution for Larval Imaging and Dissection
- For live imaging of wing discs, prepare modified HL3 media21 (70 mM NaCl, 5 mM KCl, 1.5 mM CaCl2 dyhydrate, 4 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose dyhydrate, 115 mM sucrose, 5 mM HEPES). This media allows the wing disc to be kept alive for imaging21.
- Adjust pH to 7.1 using HEPES and filter with a 0.22 μm filter. Use this media for both the wing disc dissections and as the mounting media for live imaging.
3. Preparing Slides for Wing Disc Mounting
- Place two pieces of colorless double-sided tape widthwise across a microscope slide, leaving a space of about 5 mm between them. The pieces of tape should be long enough that the ends of the tape lie flush with the edges of the microscope slide.
- Use a flat edge (such as the handle end of a pair of dissecting forceps) to press the tape firmly to the slide so that no media can seep underneath it.
- Place 25 μL of modified HL3 media between the two pieces of tape. The wing disc will be mounted in this drop of media between pieces of tape so that the tape prevents the coverslip from crushing the disc (Figure 1A).
4. Dissecting and Mounting Wing Discs for Imaging
- Dissect larvae in the prepared modified HL3 media26.
- Gently tear larvae in half using two pairs of forceps and discard the posterior half of the larva.
- Grasp the middle of the anterior half of the larva with one pair of forceps and use another pair of closed forceps to push the mouth hooks back into the larva until the larva is completely inverted.
- The wing discs can be most easily found by looking for the sharp bends in the two darker colored primary branches of the tracheae that run down both sides of the larvae. The wing discs lie directly under these bends in the tracheae in an inverted larva.
- Gently remove the wing discs and transfer them to the 25 μL of HL3 media on the prepared microscope slide. It is important to make sure that no pieces of trachea are left attached to the dissected wing discs as this can cause the discs to float, making imaging difficult.
- Arrange the wing disc so that the peripodial side of the wing pouch is facing upward away from the microscope slide with the disc lying flat (see Figure 1A,B).
- Cover the wing disc and tape with a coverslip and seal the coverslip using nail polish. Once the polish is dry, immediately proceed to the imaging steps below.
5. Imaging Dpp Release
- Image the mounted wing disc using a confocal laser scanning microscope with a 40x oil objective and 488 nm laser. The Dpp-GFP should appear as a stripe of GFP fluorescence down the center of the wing disc with small puncta of Dpp-GFP being released from this center stripe of cells.
- Collect images at a speed of 2 Hz for 2 min to visualize Dpp release. For best results, set the laser at a setting just strong enough to get a clear image of the Dpp-releasing cells to avoid over-bleaching during image collection. The images can be collected as a time series which can then be exported as AVI files (no compression, 3 frames/s) to obtain video files of the Dpp release.
Representative Results
Figure 2 shows representative live-imaging results of this protocol. When the protocol is successful, Dpp-GFP can be seen as a stripe down the center of the wing disc with nuclei visible as non-fluorescent circles within the Dpp-GFP region (Figure 2). Dpp-GFP release is visible as fluorescent puncta that appear and disappear. We have observed Dpp-GFP fluorescence appearing and disappearing in the cell bodies and far from the cell bodies. Dpp signaling is dependent on actin-based filopodia-like structures called cytonemes that extend far from the cell body22,23. Therefore, it is likely that Dpp-GFP puncta that are visualized far from the Dpp-producing cell bodies in these presented videos are likely in cytonemes or at cytoneme “synapses”15. These puncta are most apparent close to the D/V boundary, which can be seen as the gap in the Dpp-GFP stripe.
Figure 3 shows a suboptimal result. In the method described here, the wing discs are mounted so that the peripodial side faces away from the microscope slide so that the disc is imaged from the peripodial side (as shown in Figure 1B). If the wing disc is imaged from the reverse side, that is, peripodial side toward the microscope slide, the Dpp-GFP fluorescence will appear out of focus and resolution will be poor, resulting in the suboptimal result shown in Figure 3. Step 4.6 is therefore critical to ensure that the wing disc is lying flat in the correct orientation before imaging to avoid this result.
Figure 4 shows a time series of images of a Dpp>GFP (dpp-GAL4; UAS-GFP) third instar larval wing disc. When GFP is not fused to Dpp, we do not observe the appearance of puncta outside of the cell bodies. This control is important for the conclusion that Dpp-GFP behaves differently than GFP alone.
Figure 5 shows a time series of images of a dpp-GAL4 driver alone third instar larval wing disc. Images were taken with the same microscope acquisition settings as all of the other samples. These images show that fluorescent puncta do not appear when Dpp-GFP is not expressed.
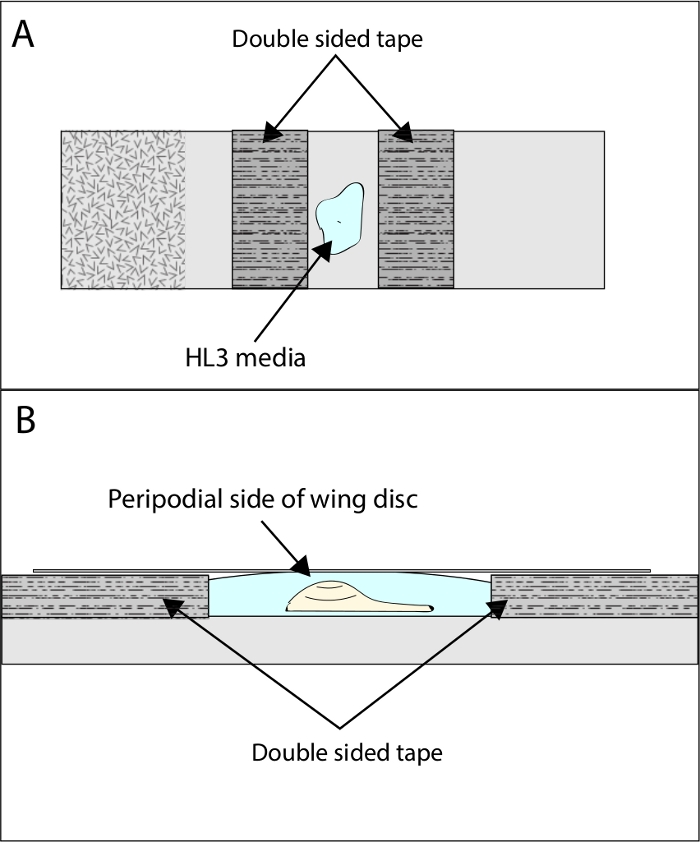
Figure 1: Illustration of wing disc mounting setup. To image Dpp-GFP release, the dissected wing discs should be mounted as shown. (A) Two pieces of double-sided tape are placed widthwise across the microscope slide with the wing disc mounted in modified HL3 media between the tape. (B) Side view of a correctly mounted wing disc. The disc is oriented so that the peripodial side of the wing pouch faces upward towards the coverslip. Please click here to view a larger version of this figure.
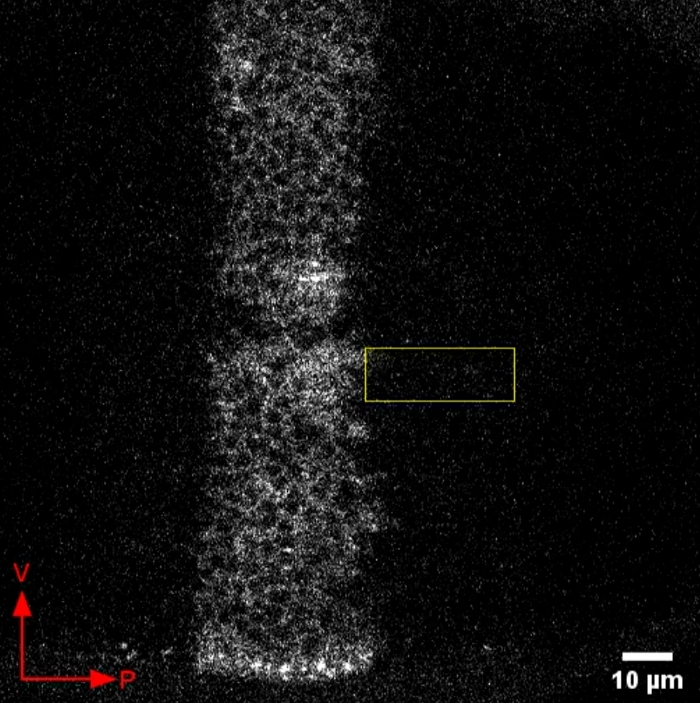
Figure 2: Representative video of Dpp-GFP release in the wing disc. Dpp-GFP was expressed in the wing disc using the UAS-GAL4 system. Shown here is a representative video of live imaging results of Dpp-GFP release. Cell nuclei can be seen as circular gaps in fluorescence. Secreted Dpp-GFP can be seen as bright puncta. Due to the contrast, these bright puncta are easiest to observe outside of the region that includes the Dpp-producing cell bodies. Such an area is indicated by a rectangle that disappears after the first few frames. Ventral and posterior directions of the wing disc are indicated by the arrows. Please click here to download this video.
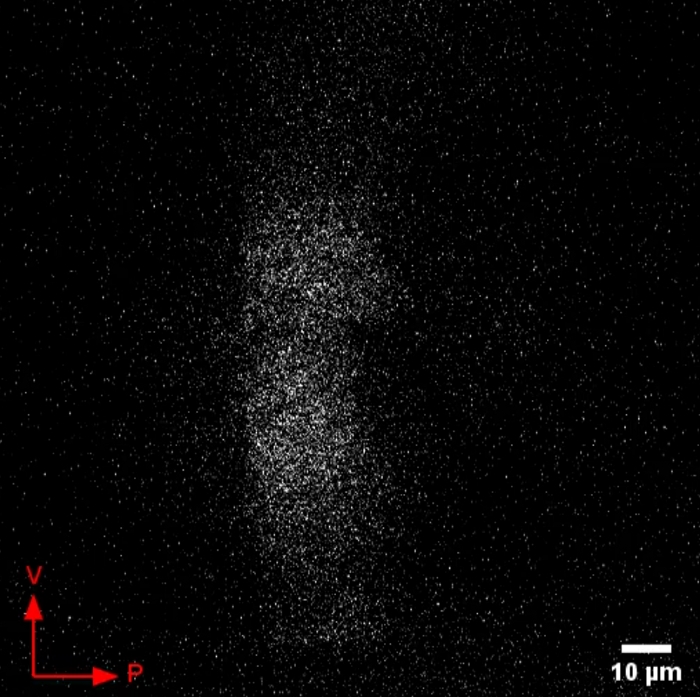
Figure 3: Suboptimal result of Dpp-GFP release imaging. Example of a suboptimal result which occurs if the wing disc is orientated peripodial side face-down. Due to the incorrect orientation of the wing disc pouch Dpp-GFP is not in focus, clear nuclei cannot be seen, and resolution remains poor no matter how the parameters of the microscope are adjusted. Ventral and posterior directions of the wing disc are indicated by the arrows. Please click here to download this video.
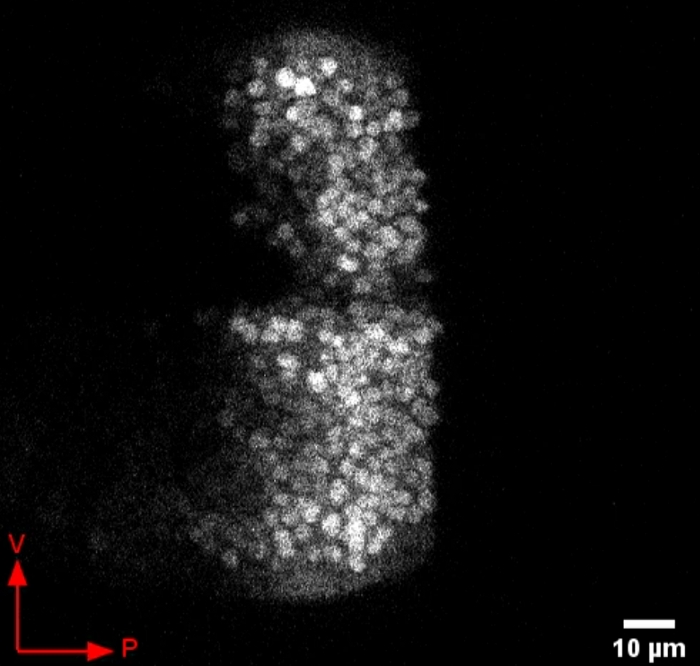
Figure 4: Representative video of GFP expression in Dpp-producing cells of the wing disc. GFP expression appears brightly in the cell bodies and does not appear as bright puncta released in the periphery. Ventral and posterior directions of the wing disc are indicated by the arrows. Please click here to download this video.

Figure 5: Representative video of dpp-Gal4 third instar wing discs without the expression of Dpp-GFP or GFP. If Gal4 cannot drive expression of GFP or Dpp-GFP due to lack of UAS-GFP or UAS-Dpp-GFP, no fluorescence is observed. Please click here to download this video.
Discussion
BMPs such as Dpp make their significant impact when they bind a complex of membrane bound receptors to cause a cascade of intracellular signaling in neighboring or apparently distant cells. Dr. Thomas Kornberg’s lab has shown that cells that produce the Dpp signal contact cells that receive the signal using actin based thin filapodia-like structures called cytonemes15,24,25. These data suggest that developmental cell-cell communication in this context may be similar to a neuronal synapse26. In synaptic communication, the dynamics of neurotransmitter release are crucial to its effect on the postsynaptic cell. Similarly, the dynamics of Dpp release are also important for its downstream consequences12. Therefore, it is important to be able to measure the dynamics of Dpp release in different genetic backgrounds and conditions to understand what factors impact Dpp release.
Here we have presented the optimized method to image Dpp release. We have also tried using wells to culture the wing disc for imaging. Too deep of a well can result in difficulty focusing upon the Dpp release events. In addition, the wing disc can move during live imaging, which complicates analysis. Double sided tape allows enough space between the cover slip and slide to prevent crushing of the wing disc, while allowing proper focus. We have found that turning the microscope on a half an hour before imaging to allow the microscope to warm up prevents drift of the image over the duration of the video. Placement of the wing disc with the peripodial side of the wing pouch facing upward is very important for imaging Dpp release. We found that the opposite orientation does not allow us to image Dpp release events.
This method could be used to look for genetic modifiers of Dpp release. We have observed that inhibition of an ion channel impacts Dpp release12. Other ion channels could also affect Dpp release dynamics. We could also test how environmental conditions such as teratogens affect release of Dpp. Modifiers of Dpp release may reveal the molecular mechanism that controls ligand secretion. Release dynamics may be important for the downstream consequences of several different developmental signaling ligands. While we have only described how we image Dpp release, other ligands such as Hedgehog have been observed in cytoneme mediated signaling and could be regulated by a similar mechanism.
開示
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Sarala Pradhan for working on an earlier version of this protocol. We would like to thank NSF-IOS 1354282 for funding while we developed this protocol. We would like to thank NIH-NIDCR RO1DE025311 for funding our lab currently.
Materials
| Baker's yeast | Red Star | ||
| CaCl2 dyhydrate | Fisher Scientific | C79-500 | |
| Coverslips | VWR | 484-457 | |
| Double-sided tape | Scotch | ||
| Drosophila Agar Type II | Apex | 66-104 | |
| Drosophila melanogaster: Dpp-GAL4/TM6 Tb Hu | This stock will soon be made available at Bloomington Drosophila Stock Center | ||
| Drosophila melanogaster: Sp/CyO-GFP; UAS-Dpp-GFP/TM6 Tb Hu | This stock will soon be made available at Bloomington Drosophila Stock Center | ||
| Dumont Tweezers #5 | World Precision Instruments | 500233 | Forceps for dissecting |
| HEPES | Sigma Aldrich | H3375 | |
| KCl | Fisher Scientific | AC193780010 | |
| Light Corn Syrup | Karo | ||
| Malt Extract | Breiss | ||
| MgCl2 | Fisher Scientific | AC223210010 | |
| Microscope slides | Sigma Aldrich | S8400 | |
| NaCl | Fisher Scientific | S271-500 | |
| NaHCO3 | RPI | S22060-1000.0 | |
| Nail polish | Electron Micsroscopy Sciences | 72180 | |
| Propionic Acid | VWR | U330-09 | |
| Soy Flour | ADM Specialty Ingredients | 062-100 | |
| Sucrose | Fisher Scientific | S5-3 | |
| Sucrose | Fisher | S512 | |
| Tegosept | Genesee Scientific | 20-259 | |
| Trehalose dyhydrate | Chem-Impex International, Inc. | 00766 | |
| Yellow Corn Meal | Quaker | ||
| Zeiss LSM 780 confocal microscope | Zeiss | Microscope for live imaging | |
| Zeiss SteREO Discovery.V8 microscope | Zeiss | Microscope for dissections |
参考文献
- Ferguson, E. L., Anderson, K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 71 (3), 451-461 (1992).
- Ferguson, E. L., Anderson, K. V. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 114 (3), 583-597 (1992).
- Wharton, K. A., Ray, R. P., Gelbart, W. M. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 117 (2), 807-822 (1993).
- Raftery, L. A., Twombly, V., Wharton, K., Gelbart, W. M. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. 遺伝学. 139 (1), 241-254 (1995).
- Raftery, L. A., Sanicola, M., Blackman, R. K., Gelbart, W. M. The relationship of decapentaplegic and engrailed expression in Drosophila imaginal disks: do these genes mark the anterior-posterior compartment boundary. Development. 113 (1), 27-33 (1991).
- Blackman, R. K., Sanicola, M., Raftery, L. A., Gillevet, T., Gelbart, W. M. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 111 (3), 657-666 (1991).
- de Celis, J. F. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 124 (5), 1007-1018 (1997).
- De Celis, J. F. Pattern formation in the Drosophila wing: The development of the veins. Bioessays. 25 (5), 443-451 (2003).
- Letsou, A., et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 80 (6), 899-908 (1995).
- Nellen, D., Affolter, M., Basler, K. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell. 78 (2), 225-237 (1994).
- Raftery, L. A., Sutherland, D. J. TGF-beta family signal transduction in Drosophila development: from Mad to Smads. 発生生物学. 210 (2), 251-268 (1999).
- Dahal, G. R., Pradhan, S. J., Bates, E. A. Inwardly rectifying potassium channels regulate Dpp release in the Drosophila wing disc. Development. 144 (15), 2771-2783 (2017).
- Dahal, G. R., et al. An inwardly rectifying K+ channel is required for patterning. Development. 139 (19), 3653-3664 (2012).
- George, L. F., et al. Ion Channel Contributions to Wing Development in Drosophila melanogaster. G3. 9 (4), 999-1008 (2019).
- Huang, H., Liu, S., Kornberg, T. B. Glutamate signaling at cytoneme synapses. Science. 363 (6430), 948-955 (2019).
- Teleman, A. A., Cohen, S. M. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 103 (6), 971-980 (2000).
- Miesenbock, G., De Angelis, D. A., Rothman, J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 394 (6689), 192-195 (1998).
- Dahal, G. R., Pradhan, S. J., Bates, E. A. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development. 144 (15), 2771-2783 (2017).
- Duffy, J. B. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 34 (1-2), 1-15 (2002).
- Hazegh, K. E., Reis, T. A Buoyancy-based Method of Determining Fat Levels in Drosophila. Journal of Visualized Experiments. (117), e54744 (2016).
- Feng, Y., Ueda, A., Wu, C. F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. Journal of Neurogenetics. 18 (2), 377-402 (2004).
- Hsiung, F., Ramirez-Weber, F. A., Iwaki, D. D., Kornberg, T. B. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 437 (7058), 560-563 (2005).
- Roy, S., Hsiung, F., Kornberg, T. B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 332 (6027), 354-358 (2011).
- Kornberg, T. B., Roy, S. Cytonemes as specialized signaling filopodia. Development. 141 (4), 729-736 (2014).
- Roy, S., Huang, H., Liu, S., Kornberg, T. B. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 343 (6173), 1244624 (2014).
- Kornberg, T. B., Roy, S. Communicating by touch–neurons are not alone. Trends in Cell Biology. 24 (6), 370-376 (2014).

