A Semi-high-throughput Imaging Method and Data Visualization Toolkit to Analyze C. elegans Embryonic Development
概要
This work describes a semi-high-throughput protocol that allows simultaneous 3D time-lapse imaging of embryogenesis in 80–100 C. elegans embryos in a single overnight run. Additionally, image processing and visualization tools are included to streamline data analysis. The combination of these methods with custom reporter strains enables detailed monitoring of embryogenesis.
Abstract
C. elegans is the premier system for the systematic analysis of cell fate specification and morphogenetic events during embryonic development. One challenge is that embryogenesis dynamically unfolds over a period of about 13 h; this half day-long timescale has constrained the scope of experiments by limiting the number of embryos that can be imaged. Here, we describe a semi-high-throughput protocol that allows for the simultaneous 3D time-lapse imaging of development in 80–100 embryos at moderate time resolution, from up to 14 different conditions, in a single overnight run. The protocol is straightforward and can be implemented by any laboratory with access to a microscope with point visiting capacity. The utility of this protocol is demonstrated by using it to image two custom-built strains expressing fluorescent markers optimized to visualize key aspects of germ-layer specification and morphogenesis. To analyze the data, a custom program that crops individual embryos out of a broader field of view in all channels, z-steps, and timepoints and saves the sequences for each embryo into a separate tiff stack was built. The program, which includes a user-friendly graphical user interface (GUI), streamlines data processing by isolating, pre-processing, and uniformly orienting individual embryos in preparation for visualization or automated analysis. Also supplied is an ImageJ macro that compiles individual embryo data into a multi-panel file that displays maximum intensity fluorescence projection and brightfield images for each embryo at each time point. The protocols and tools described herein were validated by using them to characterize embryonic development following knock-down of 40 previously described developmental genes; this analysis visualized previously annotated developmental phenotypes and revealed new ones. In summary, this work details a semi-high-throughput imaging method coupled with a cropping program and ImageJ visualization tool that, when combined with strains expressing informative fluorescent markers, greatly accelerates experiments to analyze embryonic development.
Introduction
The C. elegans embryo is an important model system for mechanistic cell biology and analysis of cell fate specification and morphogenetic events driving embryonic development1,2,3,4,5,6,7,8,9. To date, much of the characterization of both cellular-level events and cell fate specification in the embryo has been achieved using relatively high temporal resolution one-at-a-time imaging experiments (i.e., acquisition every 10–100 s) of embryos expressing fluorescent markers. Although well suited for events on the order of seconds to tens of minutes, this approach becomes technically limiting for the characterization of longer processes, on the order of hours to days. Embryonic development from first cleavage to the end of elongation takes about 10 h. At this time-scale, semi-high-throughput methods that would allow for simultaneous lower time resolution imaging (i.e., acquisition at 5–20 min time intervals) of larger cohorts of embryos, from different conditions, would open up a new range of experiments; for example, enabling systematic large-scale screening efforts and the analysis of sufficient numbers of embryos for comparisons of the consequences of molecular perturbations.
Here, we describe a semi-high-throughput method for monitoring C. elegans embryogenesis that enables the simultaneous 3D time-lapse imaging of development in 80–100 embryos, from up to 14 different conditions, in a single overnight run. The protocol is straightforward to implement and can be carried out by any laboratory with access to a microscope with point visiting capabilities. The major steps in this protocol are outlined in Figure 1. In brief, embryos are dissected from gravid adults expressing fluorescent markers of interest and transfer young embryos (2–8 cell stage) to wells of a 384-well plate for imaging. In this format, the relatively small well size funnels embryos into a narrow area, which facilitates the identification of fields containing multiple embryos for time-lapse imaging. To maintain roughly synchronous development across the cohort of embryos, dissections are performed in chilled media and the plate is held on ice, which prevents significant development during the hour-long dissection time window. The plate is transferred to the microscope and embryos are filmed in a temperature-controlled room overnight, at 20 min time intervals, using a 60x oil immersion 1.35 NA lens, to collect the full z-range in 2 µm steps. Fifty fields, each containing between 1–5 embryos, are imaged in a single overnight run. Depending on the desired experiment, the time resolution could be increased (for example, imaging at 5–10 min intervals) by proportionally decreasing the number of imaged fields.
With this protocol, even a single overnight run generates a significant amount of data (80–100 embryos spread out over 50 fields) and larger experiments can quickly become unmanageable with respect to data analysis. To facilitate processing, visualization and streamline analysis of this data, a program was built to crop out and orient embryos and perform pre-processing steps (optional), and an ImageJ macro that compiles the data to simplify viewing. These programs can be used to process images collected using conventional approaches, as they are independent of the imaging method, requiring only a single brightfield plane. The first program takes in a 4D field containing multiple embryos (GUI option or source code embryoCrop.py) or multiple 4D fields containing multiple embryos (screenCrop.py), tightly crops embryos and orients them in an anterior-posterior configuration. These programs also give users the option to perform background subtraction, drift correction, and attenuation correction. The resulting files are uniformly pre-processed, tightly cropped tiff stacks for each embryo that are amendable to automated image analysis. To make it simpler to view all embryos for each condition, an ImageJ macro (OpenandCombine_embsV2.ijm) was written, which assembles all embryos from a given condition into a single tiff stack and arrays brightfield images and maximum intensity projection color (RGB) overlays, side-by-side, for each embryo. The methods were validated by using them to characterize embryonic development after knock-down of 40 previously-described developmental genes in a pair of custom-built strains expressing fluorescent markers optimized to visualize key aspects of germ-layer specification and morphogenesis10,11. Together, the semi-high throughput embryo imaging protocol and image processing tools will enable higher sample number experiments and large-scale screening efforts aimed at understanding developmental processes. In addition, these strains will also provide an efficient means for examining the effects of molecular perturbations on embryogenesis.
Protocol
1. Preparing C. elegans Embryos for Semi-high-throughput Imaging
NOTE: The goal of this portion of the protocol is to load a population of semi-synchronized (2 to 8-cell stage) C. elegans embryos, dissected from suitable marker strains (Figure 2), into a glass-bottom 384-well plate for imaging. Other plate formats could also work, but the 384 well plates are preferred because the small well size constrains the spread of embryos to a relatively small area, which facilitates the identification of fields containing multiple embryos for time-lapse imaging. Roughly synchronizing the embryos ensures that the full course of development is captured for each of the embryos in a field.
- Prepare 5–10 mL of 0.1 mg/mL solution of tetramisole hydrochloride (TMHC) anesthetic dissolved in ice cold M9 medium (0.45 M Na2HPO4∙7H2O, 0.11 M KH2PO4, 0.04 M NaCl, 0.09 M NH4Cl) to use during dissection and imaging. This media includes an anesthetic to ensure that moving hatched larva do not disrupt imaging of embryos at earlier developmental stages.
NOTE: If using the cropping and visualization tools to analyze data acquired using standard agarose pad mounting methods, skip ahead to section 3 below. - Aliquot 70 µL of the prepared solution into individual wells of a glass-bottom 384-well plate with one well for each condition; avoid the outer two rows of the plate to prevent edge effects.
NOTE: It is useful to mask surrounding wells using adhesive PCR plate foil (see example in Figure 1D) this preserves adjacent wells for future experiments and makes it easier to locate appropriate wells under the dissection microscope. Once the solution is aliquoted, keep the plate and remaining solution on ice throughout dissection. - Generate mouth pipets for transferring embryos from the depression slide (where the gravid hermaphrodites are dissected) to the wells. Pull capillary pipets (25 µL calibrated pipets) over a flame and break to generate a fine tapered end (Figure 1D). One pipet is needed per condition to prevent cross-contamination; discard pipet after use.
- Use fine tweezers to transfer ~10 gravid adults under a dissection scope into 150 µL of the ice cold TMHC solution aliquoted onto a depression slide for each condition. Using the tweezers and a scalpel, dissect the worms to release the embryos.
- Load a pulled capillary pipet into the aspirator attachment included with the pipets, and mouth pipet to transfer all of the 2 to 8-cell-stage embryos into an individual well of the prepared plate (Figure 1D). Avoid transferring late stage embryos and dissection debris. Examine under a dissection scope and if aggregates of embryos are present, mouth pipet up and down or tap clumps of embryos with pipet tip to disperse.
NOTE: To prevent cross-contamination, clean dissection equipment and use a fresh mouth pipet while moving between conditions. Store plate on ice between dissections. - Once worms from all conditions have been dissected and embryos placed in their appropriate well, spin the 384-well plate for 1 min at 600 x g to settle the embryos.
- Wipe the bottom of the plate with an ethanol-soaked wipe to remove any residue and place the plate on a confocal microscope equipped with a plate holder in a temperature-controlled environment.
NOTE: The steps that follow detail this method using the lab setup; please modify acquisition conditions to suit experimental needs and equipment. Here, a confocal scanner box equipped with a microlens-enhanced dual Nipkow spinning disk, a 512 x 512 EM-CCD camera, a high-precision auto-XY-Stage (designated resolution 0.1 µm) and motorized z-axis control (designated resolution 0.1 µm) is used. This system is kept in a 16 °C room, which maintains the microscope temperature between 21 and 23 °C during overnight imaging. - To identify fields with suitable embryos, perform a pre-scan of each well using a 10x 0.4 NA objective and suitable imaging software. The most optimal fields will contain more than one early stage embryo that is in the same focal plane as other embryos and ideally will have minimal contact between adjacent embryos. Mark positions of suitable regions.
- To select imaging fields, switch to the 60x objective and adjust the focal plane at each point visit to appropriately section the embryos. 1–4 fields per well are imaged, for a total of 50 fields across 14 wells, and each field can contain between one and five embryos. Overall, 4 to 15 embryos are selected from each condition for high-resolution imaging.
NOTE: A key variable at this step is the use of sufficient oil; place oil on the objective, visiting points in each well to spread the oil around the surface of all of the wells and then apply an additional drop of oil to the objective prior to selection of fields. - Image the selected fields using a 60x 1.35 NA objective to acquire 18 z sections at 2 µm intervals every 20 min for 10 h. The imaging conditions using the germ-layer and morphogenesis reporter strains are as follows: brightfield, 90% power, 25 ms, 20% gain; 488 nm, 100% power, 200 ms, 60% gain; 568 nm, 45% power, 150 ms, 60% gain.
- After imaging is complete, assess embryonic lethality by performing a low magnification (10x 0.4 NA objective) whole well brightfield scan ~20–24 h following the start of overnight imaging.
2. Embryonic Lethality Scoring
- Using the post-run 10x scanned fields, assess embryonic lethality and larval defects by counting hatched worms and unhatched embryos for each well.
- Score unhatched embryos as embryonic lethal. Exclude arrested one- to four-cell-stage embryos from lethality count, since young dissected embryos sometimes fail to complete eggshell formation (if meiosis II is not yet complete) and permeability defects can lead to osmotic complications during the first two divisions.
- Score partially hatched or fully hatched worms with body morphology or behavioral defects, such as dumpy or paralyzed, as 'abnormal larva'.
3. Automated Cropping (Figure 3A)
NOTE: The software is housed in two locations: (1) Zenodo houses a user-friendly version of the software12 that does not require any programming expertise. (2) Github contains the source code for our embryoCropUI.py and screenCrop.py software13, which require proficiency with Python. Detailed instructions for downloading and operating both versions of the program can be found below.
- Automated cropping using embryoCropUI executable version (user friendly version)
- To use the embryoCropUI program, first download the program from Zenodo (https://zenodo.org/record/3235681#.XPAnn4hKg2w)12.
- Download the MacOS or Windows format version of the program (note that the MacOS version requires MacOS X10.11 or higher).
- Download the instructions file (GUI_Instructions_zenodo_repoV2.docx), which provides step by step instruction for testing and using the embryoCropUI program.
- Download the test_files.zip to test if the program is properly functioning on the platform (see instructions).
- Once downloaded, unzip and navigate to find the embryoCropUI executable (…embryoCropUI_WINDOWSembryoCropUIembryoCropUI.exe) or (…embryoCropUI_MacOSembryoCropUIembryoCropUI.exe). Double click to launch (or chose 'open with' terminal) and run the embryoCropUI executable.
- The GUI executable crops one 4D field of view at a time. In the upper left-hand corner, select the open button to load the specific field to crop (multi-tiff). If cropping a tiff series, with multiple dimensions (i.e., z, time, channel), load only the first image in the series within the folder (one tiff series per folder).
- Once images have been loaded, specify the following information: number of z- slices, (Z), number of time points (T), number of channels (C), the channel that corresponds to DIC or brightfield (first=1, second=2, etc.).
- Select additional processing to run on the images. The program offers Drift Correction, Background Subtraction, and Attenuation Correction.
- Specify parameters for Background Subtraction and Attenuation Correction to guide processing efforts in the GUI prompt. For Background Subtract, define the largest feature size to reflect the size of meaningful signal; this feature size should not be considered as background and must be an odd number value. Input a value from 0-1 for Attenuation Correction. The Attenuation Correction value reflects the percent of original intensity that remains at the furthest depth of the object being imaged.
NOTE: Background Subtraction and Attenuation Correction must be run together. - Specify the image collection order (i.e., channel-z-time (czt), or z-channel-time (zct)).
- Specify the microns per pixel for the images based on the camera being used.
NOTE: Poor image cropping will occur if pixel size is not properly defined. - Select Run at the bottom left corner. A new subfolder labeled “crop” will be created in in the same path as the uncropped folder; cropped versions will be saved in this location. Depending on file size, cropping should complete within seconds to minutes.
- To use the embryoCropUI program, first download the program from Zenodo (https://zenodo.org/record/3235681#.XPAnn4hKg2w)12.
- Automated cropping using screenCrop.py (batch version alternative to embryoCrop GUI described above; Python savvy users only)10,13
- To use screenCrop.py, the Python software version for cropping of larger data sets in a batch format, clone or download the source code from Github (github.com/renatkh/embryo_crop.git).
- Read the instructions for configuring a proper virtual environment and follow the file-naming system described; both of which are detailed in the README file in the Github repository: https://github.com/renatkh/embryo_crop/blob/master/README.md.
- Once environmental variables and naming conventions have been properly established, open parameters.py and screenCrop.py in an editor of choice.
- To modify adjustable parameters without touching the source code, edit the parameters.py file.
NOTE: it is also possible to directly change parameters within the header of screenCrop.py.- If using the parameters.py configuration file, change the use_configure setting to True. If using direct editing within screenCrop.py, leave the use_configure setting at False.
- Locate the following information within the parameters.py file and make modifications to suit desired imaging parameters and file structure:
- loadFolder (line 9): Change to the drive on which the files are stored (e.g., Z:/, D://, etc.)
- date (line 7): Change to the folder containing files for the imaging session. This is referred to as 'Experiment Folder Name' in the CSV tracking file (see instructions).
- trackingFile (line 11): Change to the path to the CSV file in which experiment information is stored.
- z (line 13): Set as the number of z planes.
- nT (line14): Set as the number of timepoints.
- nWells (line 19): Set as the number of wells used.
- pointVisits (line 20): Set as the maximum number of point visits (per well).
- In line 10 find the location currently occupied by 'CV1000/' and input the outer folder used in the file path. To avoid issues, use the following convention: 'XXXXXXX/'.
- In Line 12, input a valid file path for storing aspect ratio data for cropped data.
- In Lines 15, 16, 17, and 18 input True/False for whether the images go through the following processing:
- Input True or False for drift correction on Line 15.
- Input True or False for background subtract on Line 16. Feature size must be empirically determined for different marker strains and pixel sizes. In the configuration here, feature size was defined as 41 for the Germ-Layer strain and 201 for the Morphogenesis strain. Background subtract must be done in conjunction with attenuation correction.
- Input True or False for attenuation correction on Line 17.
- Input True or False for anterior posterior rotation on Line 18.
- Once all changes have been made, cropping can begin. To do this, switch to screenCrop.py and select the play icon in the toolbar, in the drop-down menu select Run As > Python Run.
- As cropping can take several hours to complete for a large dataset (50 point visits 18 z steps, 3 channels, 31 timepoints), track progress of cropping in the console window. Once cropping is completed, a small window will show previews of the cropped images before saving. For each image there are 3 options:
- Save: Press Space Bar to save the image if the image is cropped properly with no areas of interest being cut off.
- X: Press X if the image appears to have areas of interest cut off; the image will be saved with an X in front of the name to separate it from the other images.
- Delete: Press D to delete the cropped image if the image is not cropped properly or the embryo is not satisfactory.
NOTE: The images will be saved to a subfolder named "Cropped" in the Load Folder (defined in Line 9 of the program).
4. Visualization (Figure 4)
NOTE: OpenandCombine_embsV2.ijm10,12 is an ImageJ macro that will construct an easy to view tiff file from all the images for a specific strain and condition. Installation of FIJI/ImageJ14,15 is required. This macro runs according to our file structure; it will need to be modified to work with other file structures. A guide to proper file structure and detailed description of important considerations can be found at the end of the GUI_Instructions_zenodo_repoV2.docx file on the Zenodo repository. Please read through these instructions completely before imaging to properly name and structure files to interface best with this macro. For reference, our file location structure looks like this:
Z:croppedTargetStrainEmb#Target_Emb#_15 Digit Unique Identifier _W##F#_T##_Z##_C#.tif
i.e. Z:croppedEMBD0001GLSEmb1EMBD0001_Emb1_20140327T135219_W02F1_T01_Z01_C1.tif
- Download OpenandCombine_embsV2 and GUI_Instructions_zenodo_repoV2.docx from Zenodo (https://zenodo.org/record/3235681#.XPAnn4hKg2w).
- Prepare the following information:
-The location where the image folders are stored (following cropping).
-The image folder name(s) for the specific condition(s) to be processed (i.e., EMBD0002). This is referred to as "Target" in the above example
-A 15 digit alpha-numeric unique identifier that is specific to a given overnight experiment—this identifier is the data acquisition folder name (i.e., 20140402T140154) and the unique identifier is embedded in the individual tiff file name (EMBD0002_Emb1_20140402T140154_W06F2_T01_Z01_C1) for every embryo imaged on that day. The macro uses this identifier to check for embryos acquired on the same date and can assemble repeated conditions, with separate dates, into separate ImageJ files. - Open ImageJ, and drag-and-drop the macro file, OpenandCombine_embsV2.ijm, to the ImageJ bar, or open the macro directly.
- Once the macro is open, locate lines 3 and 4. Input the information gathered in (section 4.2) according to the following steps:
- In Line 3 (RNAL), input the target name for the images to be processed. Input each target in the following structure newArray("XXXXXXXX/"); include quotations. To run multiple conditions at once, separate with a comma and keep all target names within the parenthesis (i.e., newArray("EMBD0000/","EMBD0001/","EMBD0002/").
- In Line 4 (date), input the 15 digit alpha-numeric unique identifier in quotations, for example newArray("20140402T140154").
- Press Run at the bottom left of the macro window. A window will appear, which will launch a prompt to navigate to the outer folder containing the cropped image folders (specified in 4.4.1).
- Once selected, another window will appear, which will allow specification of imaging parameters.
- Enter the number of channels, the number of Z slices, the font size for the text used in labeling the compiled images, and the color for each of the channels.
- Check on/off auto contrasting in all channels.
- Once all parameters have been specified, click OK at the bottom of the window. The composite file will begin to assemble; this will take several minutes, per condition, to complete.
- Once completed, review the files that are left open if desired. They can be closed without saving, as the macro has already saved the files to a newly created folder which is named in the following manner: outer folder name (specified in the prompt of section 4.5) + "-fiji-processed-output" (i.e., Z: cropped-fiji-processed-outputEMBD0002_GLS_20140402T140154.tif).
Representative Results
A significant challenge in characterizing the effect of molecular perturbations on C. elegans embryonic development is that it takes about 10 h for embryos to progress from first cleavage to the end of elongation at 20°16. A semi-high-throughput method in which large cohorts of embryos can be simultaneously imaged is useful for events on this time-scale because it permits imaging of multiple conditions in parallel with a sufficient ensemble size for each condition to enable quantitative analysis (Figure 1A).
Semi-high throughput imaging method and multi-marker strains
The semi-high-throughput imaging method described here (outlined in Figure 1B), employs a 384-well glass bottom plate; the multi-well format allows for up to 14 conditions to be arrayed in parallel and the small size of the wells constrains the search area, simplifying the identification of fields containing embryos for high resolution imaging. Here, a confocal scanner box was used, equipped with a microlens-enhanced dual Nipkow spinning disk, a 512 x 512 EM-CCD camera, a high-precision auto-XY-Stage (designated resolution 0.1 µm) and motorized z-axis control (designated resolution 0.1 µm). However, the protocol can be adapted for any confocal microscope with precision point-visiting capabilities and a stage adaptor that can accommodate a multi-well plate. A significant challenge in developing this method was devising a means to generate a plate of embryos at the same developmental stage so that the entirety of development can be captured for all of the embryos in each field. This is a challenge because the embryos arrayed onto the imaging plate first will continue to develop while later samples are dissected, resulting in a gradient of staging across the wells. To circumvent this issue, early stage embryos (2-8 cell stage) are selected and keep the dissection media and imaging plate on ice; this stalls embryogenesis for the dissected embryos until all conditions have been arrayed without significantly impacting embryonic viability10. To limit the time that the embryos sit on ice to 1 hour, two researchers perform the dissections simultaneously, which allows for the set-up of 14 different conditions in about 50 min. After dissection, the plate is spun at 600 x g for 1 min to sediment the embryos and wells are pre-scanned at low magnification to locate fields with groups of suitable embryos for high-magnification imaging; point visit locations are marked. Embryos are filmed in a temperature-controlled room overnight using a 60x oil immersion 1.35 NA lens. The samples are configured in a 4 well by 4 well region so that the surface area of the plate that is used in an experiment is comparable to a 22 x 22 mm2 standard coverslip, which allows for the use of a 60x oil objective. Uniform spreading of oil across this region prior to the start of imaging is critical for successful long-term image acquisition. The developing embryos are imaged in a solution containing anesthetic; this ensures that when the older embryos within the well hatch, their movement does not disrupt the position of adjacent younger embryos that are being imaged. Due to the impenetrable nature of the eggshell, the anesthetic does not affect movement prior to hatching. Under these conditions, 3D time-lapse data is collected in 3-channels for a total of 80-100 embryos in a single overnight experiment (discussed more below).
C. elegans embryonic development occurs in two phases. First, 10 rounds of cell division coupled to cell fate specification generate the three germ layers (ectoderm, mesoderm, and endoderm) over a 6 hour period17. In the following 7 h, morphogenetic events drive the formation of differentiated tissues8,16. To capture these two aspects of development, we built a pair of custom strains, the Germ Layer and Morphogenesis strains10 (Figure 2A), and used the semi-high-throughput protocol to image embryos from both strains. The Germ Layer strain expresses transgene-encoded fluorescent proteins that mark nuclei in the ectoderm, mesoderm and endoderm in red, yellow, and green. This strain contains three reporter transgenes: (1) a transgene expressing PHA-4::GFP that marks nuclei in the intestine and pharynx (green endoderm)10,18,19, (2) a transgene expressing an mCherry-histone fusion in the epidermis and in ~1/3 of neurons (red ectoderm; Figure 2A), and (3) a transgene that simultaneously expresses mCherry and GFP-tagged histones in body wall muscle (yellow mesoderm). The Morphogenesis strain has two transgenes that express: (1) a green epithelial junction marker (DLG-1::GFP) in the epidermis, and (2) an mCherry-tagged plasma membrane marker, in ~1/3 of neurons (Pcnd-1 promoter; Figure 2A). To enhance the effectiveness of RNAi, both strains were also engineered to contain a pair of RNAi-sensitizing mutations (nre-1(hd20) lin-15b(hd126)20. These were constructed with the goal of performing a large-scale RNAi-based screen of the ~2,000 genes required for embryonic development. However, this pair of strains will also constitute a broadly useful standardized platform for assessing developmental defects in mutants, and for more detailed analyses of the roles of different proteins in development.
For data collection in this semi-high throughput format, with these strains, green, red and bright-field z-series (18 x 2 µm2 intervals) are collected, every ~20 min, for a period of 10 h in a 16 °C temperature-controlled room; this maintains the microscope between 21-23 °C during the run. The 20 min time interval allows us to image 50 fields of embryos (point visits) per experiment. Users can tailor acquisitions to shorter intervals with fewer point visits, or longer intervals with more point visits to best suit their experimental objectives. For these strains, the early developmental timepoints are not imaged because the tissue specific reporters driving marker expression do not begin to express until mid-to-late gastrulation. During this early phase, wells were scanned at low magnification (10x) and fields are selected for high magnification imaging. Selecting fields containing more than one early stage embryo is recommended, but avoiding fields where embryos are partially overlapping, overly crowded, or are found at the extreme edge of the well. Following overnight 60x imaging, a low magnification (10x) whole well scan is performed to determine whether embryos hatch with normal appearance (WT), hatch with visible abnormalities (larval abnormal), or are embryonic lethal for each condition assessed (Figure 1B). This step serves as an easy alternative to setting up plates in parallel to assess lethality across a larger population; the whole well 10x post-scan provides embryonic lethality data on 50-80 embryos per condition. Using this measure to compare the population embryonic lethality data for controls between runs is a useful control that ensures consistency with respect to the health of the strains and the environmental conditions and processing steps. When imaged in combination, the two custom reporter strains provide informative readouts for most major developmental events, including cell fate specification, dorsal intercalation, epidermal enclosure, elongation, and neurogenesis (representative control images are shown in Figure 2B, also see Wang et al.10).
Data processing: automated embryo cropping and orientation
With our imaging protocol, each high-resolution imaging field contains between 1 and 5 embryos; these embryos are randomly oriented and are frequently positioned immediately adjacent to one another. Data collected using conventional embryo mounting techniques suffers from the same challenges. To prepare the data for subsequent visualization and analysis, considerable hands-on manipulation is needed to crop and orient the embryo sequences, as well as to pre-process them to subtract background, correct for drift, and compensate for signal attenuation with depth. To streamline this process, a custom embryo cropping program was built that isolates and orients each embryo from the broader field (Figure 1C, Figure 3). This program has been packaged as a user-friendly, stand-alone program with graphical user interface (GUI, compatible with data from most imaging platforms) that can take in a 3D time-lapse sequence of a field of embryos, and process it into individual tiff series for each embryo in the field (Figure 3B, available at https://zenodo.org/record/3235681#.XPAnn4hKg2w)10,12. The Python source code for the GUI (embryoCropUI.py), and a batch version of this program (screenCrop.py) are also available for experienced Python users on GitHub (https://github.com/renatkh/embryo_crop.git)10,13. The core functionality of this program is to locate, crop, and orient the embryo along the anterior-posterior axis. Simultaneously, background subtraction, attenuation correction, and drift correction can be performed on the dataset using optional adjustable settings.
Briefly, the automated cropping software iteratively detects individual embryos in a binary mask obtained from the bright-field images and sequentially crops the embryos from all channels and orients the images along the anterior-posterior axis (Figure 3B, first described in Wang et al.10). Prior to cropping, the software corrects for drift, subtracts background and performs depth attenuation correction in each fluorescence channel (optional). To obtain the binary mask, the software applies Canny edge detection21 to the bright-field images, performs maximum intensity projection of the three central planes and fills in small holes. The algorithm detects individual embryos by fitting a Cassini oval to a section of the binary mask outline (see Figure 3B, 3C). After aligning the oval along its main axis, the software measures red fluorescence intensity in each half of the isolated embryo and rotates the embryo such that red intensity is oriented towards the left, which puts the embryo anterior on the left for both of the reporter strains used in this work (Figure 3D). Piloting of this software revealed that most embryos were efficiently cropped as anticipated. Minor imaging issues, such as embryo drift, temporary focal plane loss (due to bubbles in oil), or crowded fields of embryos did not typically disrupt successful cropping. However, for fields where there were large clumps of embryos (overlapping somewhat in z), embryos that were significantly tilted within the imaging plane (in Z), long-term focal plane loss, or embryos that were partially cutoff, successful cropping was not always achieved. These issues can be easily circumvented by better field selection during the data acquisition phase. Overall, pre-processing data collected with this semi-high-throughput method or using conventional mounting methods is a useful first step for visualizing and analyzing embryonic datasets.
Validation of image collection and data processing methods: visualize embryonic development after knock-down of 40 previously-described genes
To validate this semi-high-throughput image collection method and custom cropping regime, a small RNAi screen was performed in the custom strains, directed against 40 genes with previously described functions in cell fate specification and morphogenesis (described by Wang, Ochoa, Khaliullin and colleagues10,11). To do this, dsRNA was generated against each target, injected L4 animals from each strain, waited 20-24 h, and then dissected out and imaged embryos using the protocol described above (for complete dataset10,11). Achieving developmental phenotypes by RNAi requires inhibition of both maternal and zygotic gene expression. Developmental genes can be maternally expressed, with the resulting protein products being loaded into the embryos, or zygotically expressed in the embryo, or, in many cases, both10,22,23. The time between injection and embryo filming is the critical variable to effectively targeting maternally and zygotically expressed populations; timing determines both the amount of injected dsRNA that is loaded into the embryo to prevent zygotic expression24 and the extent of maternal protein depletion. After the dsRNA injection, the maternal mRNA corresponding to the targeted gene gets degraded and does not recover over in a relevant time interval. Pre-existing protein depletion requires embryo production, which ejects the maternal protein from germline tissues by loading it into forming embryos. 20-24 h at 20 °C is the shortest incubation time that allows consistent maternal depletion; maternal depletion gets better at later timepoints (i.e., 36-42 h after injection). The amount of injected dsRNA that is loaded into the embryo dictates how effective the prevention of zygotic gene expression will be. The amount of loaded RNA peaks about 5-10 h after injection, when embryos with injected material are first fertilized, declining thereafter. In our experience, 24 h after injection is an optimal timepoint, in which maternal protein depletion and inhibition of zygotic gene expression are both effective. Analysis of phenotypes in the custom reporter strains used in this work, across the set of 40 test genes, showed that our combined protocol yielded distinct, highly reproducible signature phenotypes across a broad spectrum of genes involved in cell fate specification and morphogenesis (see Wang et al.10); the complete dataset is available11). As an example of this data, still images of four different embryos for control, pha-4(RNAi), pal-1(RNAi), and mex-3(RNAi) in the germ layer reporter strain are shown in Figure 4A. For a more comprehensive discussion of the phenotypes observed in the 40-gene RNAi screen, see Wang et al.10
ImageJ macro: Compilation and visualization of all data for a given condition
Visualization of large numbers of embryos from individual tiff stacks can be tedious and time consuming to work through. From our initial effort, >400 individual embryos were isolated using the custom automated cropping program described above. To speed up first-pass analysis, we built an ImageJ macro, which generates an array of all embryos imaged for a given RNAi condition in a given strain background (Figure 4B). For each embryo in the array, a brightfield image and a maximum intensity projection image are presented side-by-side, for each time point, to generate a composite movie file. While this macro was written to function with our file structure, it can be edited to accommodate the user’s file structure. For best results, however, users should follow the naming and file structure conventions set forth in the protocol (see Zenodo or Github README files for more specifics for the Python and ImageJ application naming conventions). To view all of the ImageJ processed data and get more in-depth analysis of the phenotypes observed for the 40-gene test set, please see the data deposited in the Dryad database10,11. This ImageJ visualization format enables rapid assessment of the overall phenotype and its penetrance, and makes it easy to flag data quality issues, such as presence of debris or loss of focal plane. Overall, the combination of the semi-high-throughput data acquisition method, cropping program and ImageJ visualization tool, combined with custom reporter strains, greatly facilitates larger scale efforts to study embryonic development.

Figure 1. Overview of high-content imaging method, data processing procedure, and essential tools. (A) A comparison of the features of the standard agarose pad-based method for mounting C. elegans embryos to the semi-high-throughput multi-well imaging approach described here. (B) Overview of the semi-high-throughput method for monitoring embryonic development. See text for details. (C) Overview of the data processing and visualization tools, which can be used on data acquired using either a conventional agarose pad-based mounting procedure or the semi-high-throughput multi-well plate-based method described herein. A single 3 channel time lapse embryo sequence from one field (left) or multiple 3 channel, 4-dimensional fields of embryo data (right) can be processed using the custom cropping program, which is compatible with data captured on most imaging platforms. The graphical user interface (GUI) version of the program is user friendly and does not require any programming expertise. The screenCrop.py application requires Python expertise, but it has added capabilities- namely that it can crop in batch format. The output of this step is tightly cropped, anterior-posterior oriented embryo tiff stacks. To visualize all data from a single condition, an ImageJ macro was built that compiles cropped, rotated tiff stacks to show a brightfield image and maximum intensity projection for each embryo at each timepoint. (D) Panel shows essential tools needed for dissection and setup of semi-high-throughput imaging format. Some figure components reproduced with permission from Wang et al.10. Please click here to view a larger version of this figure.
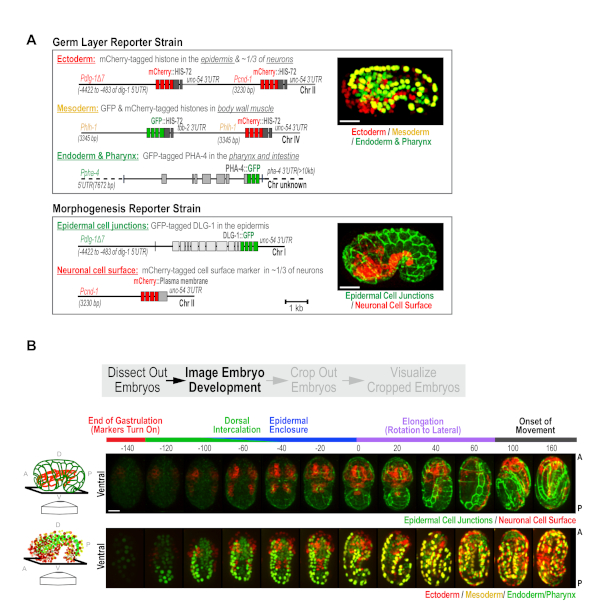
Figure 2. Custom strains generated for high-content imaging of C. elegans embryogenesis. (A) Schematics illustrate the transgenes used to construct the Germ Layer (top) and Morphogenesis (bottom) strains. (B) Maximum intensity projection panels show the developmental time-course in the Morphogenesis (top) and the Germ Layer (bottom) strains. Ventral view is shown, as illustrated in the schematics (left). Time is relative to the comma stage (t = 0), which is easily identifiable in both strains. Scale bar = 10 µm. Figure reproduced with permission from Wang et al.10. Please click here to view a larger version of this figure.
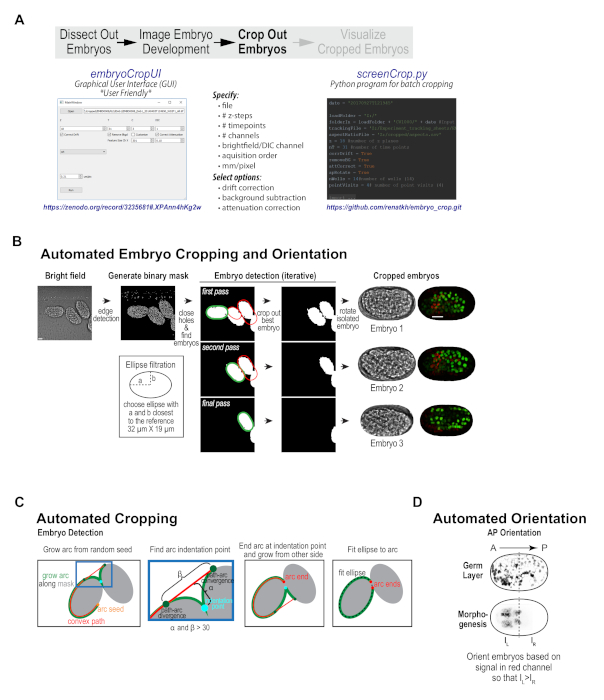
Figure 3. Custom cropping program isolates and orients individual embryos from imaging fields. (A) Schematic illustrates the features of the custom embryo cropping program and highlights the two options for accessing this program: a user-friendly GUI interface, which requires no programming expertise and is available on Zenodo (left) and a batch cropping version of the program, which requires Python experience and is available on Github (right). (B) Graphic summarizes the automated cropping algorithm — a binary mask is generated from 8-bit brightfield images and individual embryos are detected, cropped out, and oriented along the anterior-posterior axis. Scale bar is 10 µm. (C) Schematics detail the procedure used to iteratively detect embryos in the binary mask. (D) Schematic illustrates the method used for orienting embryos along the anterior-posterior axis. Panels B-D reproduced with permission from Wang et al.10. Please click here to view a larger version of this figure.

Figure 4. Custom ImageJ macro enables visualization of RNAi screening data by generating composite files for each condition. (A) Embryos for three example RNAi conditions from the 40-gene test set (see Wang et al.10,11 for complete dataset) are shown (right) that highlight the distinct signature phenotypes that are obtained for each condition and the reproducibility of the phenotypes within each condition. Panel was adapted with permission from Wang et al.10. (B) Panel illustrates how the custom ImageJ macro (OpenandCombine_embsV2.ijm) arrays embryos from a given RNAi condition into a composite ImageJ file that arrays the brightfield and maximum intensity projections for each embryo at each timepoint. While only one example is highlighted, this macro can compile the data for many RNAi conditions at once. ImageJ macro is available on Zenodo12. Scale bar = 10 µm. Please click here to view a larger version of this figure.
Discussion
This work describes a suite of tools and methods that were developed to enable larger-scale efforts to profile the function of genes in embryonic development in C. elegans. Our semi-high-throughput method allows 3D time-lapse imaging of embryonic development at 20 min resolution for 80–100 embryos in a single experiment. While this protocol can be adapted for use with any desired marker strain(s), this work demonstrates the potential of the method using two custom strains developed to monitor events during embryogenesis: (1) a germ-layer reporter strain, in which tissue specific promoters drive the expression of fluorescent histones to mark the endoderm, mesoderm and ectoderm nuclei, and (2) a morphogenesis reporter strain, which uses epidermal and neuronal promoters to drive the expression of fluorescent markers in cell-junctions and the plasma membrane. By imaging the two strains, the consequences of molecular perturbations on cell fate specification and morphogenetic events can be captured with remarkable detail. To address the challenges presented by the quantity of data generated by this method, custom tools were developed for the purpose of streamlining data processing and visualization. The first tool crops individual embryos out of a field of embryos in 3D time-lapse sequences while also rotating the embryos to a standard anterior-posterior orientation and performing background subtraction, drift correction, and attenuation correction. This program was packaged as a user-friendly GUI (https://zenodo.org/record/3235681#.XPAnn4hKg2w) and the source code and a more advanced batching version of the program for Python savvy users (github.com/renatkh/embryo_crop.git) is provided10,12,13. Finally, to visualize this processed data in a meaningful format, an ImageJ macro was devised; this allows the user to visualize all embryos from a given RNAi condition in a multipaneled movie, which shows a brightfield and maximum intensity projection for each embryo at each timepoint. Together, the strains, protocol, and data processing tools, make efforts to study C. elegans embryogenesis in larger-scale a more feasible endeavor.
While straightforward to implement on the whole, the semi-high-throughput acquisition method described here does require attention to a few critical details, which are discussed below. First, users must have access to a confocal microscope with precision point-visiting capacity that can be outfitted with a multi-well plate holder. The most critical consideration for this protocol is that the microscope be maintained in a temperature-controlled room. While many microscopes have the capacity to heat, most cannot reliably cool, thus the environmental temperature has to be adapted to maintain a constant C. elegans friendly temperature. To achieve this, the imaging room is held at 16 °C. The sample area of the instrument heats up to 21-23 °C during imaging. Fluctuations in room temperature can affect developmental timing and subtly alter the focal plane accuracy during point visiting and should be avoided as much as possible. Note that running at temperatures above 23 °C can result in a considerable spike in embryonic lethality for control conditions and is not advisable. Another key consideration is embryo selection and dispersion during dissection. Care should be taken to avoid transferring older embryos into the imaging wells, since, upon hatching animals tend to swish adjacent embryos out of view; the incidence of this is lessened by the inclusion of anesthetic in the media. The dispersion of embryos, to avoid large clumps of embryos, is also a critical variable. Clumping tends to occur when embryos are transferred to the wells in capillary pipets that are too thinly drawn out, or when embryos are not sufficiently dispersed during dissection. Another consideration is that point selection and focal plane tuning takes time. The plate is not held on ice during this process, so the embryos begin developing immediately. For the custom marker strains used in this study, there is about a 90 min window after the plate is removed from ice to accomplish plate scanning and point selection before markers begin to be expressed. This is sufficient time for set up on our instrument with these strains, but other microscopes and strains may offer additional time challenges that will require troubleshooting. Finally, the method we describe employs a 60x oil objective to collect z-stacks of 50 fields at 20 min time intervals. As we highlight, the temporal resolution can be increased by decreasing the number of fields imaged. For example, 10 fields can be imaged at 4 min time resolution. A second alternative for increasing temporal resolution is to use a lower magnification, high numerical aperture objective; in this case, the larger field of view allows imaging of a large number of embryos at higher time resolution with only a moderate reduction in spatial resolution.
To enable data processing and visualization on a larger scale, we built custom tools and made them freely available. The most broadly useful tool is the custom cropping GUI, which requires no programming expertise to operate. The GUI can be used to crop out and orient embryos at all stages of development and is compatible with all markers, making it useful not only for developmental biologists, but also for researchers studying processes in the early embryo. Because the output of the GUI is precisely cropped, oriented, scaled, drift-corrected data, the data can be easily funneled into an automated analysis pipeline, making this tool useful for analysis of past and future data; if appropriate strains are used, resulting data files can be used as input for existing automated analysis regimes, such as embryogenesis alignment tools25. In order to use the program, it is important for users to be aware that the cropping algorithm requires a brightfield or DIC image to be collected for each step and timepoint. In addition, users should note that the anterior-posterior orientation algorithm is structured based on the distribution of the red signal in the germ-layer reporter and morphogenesis report strains, shown in this work. Thus, accuracy of AP orientation in other marker strains will need to be evaluated on a case-by case basis. The GUI has been tested with data collected on three different confocal imaging platforms and is compatible across the board for multi-tiff stacks and tiff series. In addition to the user-friendly version, the source code for the GUI and a batch version of this program has also been made available, with the caveat that programming experience is required, and file structures and naming conventions will need to be followed. Finally, an ImageJ processing macro was built to take raw image stacks for all embryos, in each RNAi condition, and compile an informative array to show all embryos for that condition in brightfield and fluorescence maximum intensity projection at each time point. This tool can be adapted to user’s specifications and is a useful macro to make data exploration and quality control assessment simpler.
Together, the semi-high-throughput filming method along with the embryo cropping and image processing tools described herein will facilitate analysis of C. elegans embryonic development using a variety of mutants, perturbations, and marker strains. In our own lab, a large-scale screen employing these methods to film embryos from the two custom engineered strains described here after knockdown of ~2,000 genes required for development, is currently underway. Finally, the data from this effort will serve as a further resource to enhance efforts to understand cell fate specification and morphogenetic events during embryogenesis.
開示
The authors have nothing to disclose.
Acknowledgements
S.D.O. was supported by the National Institute of General Medical Sciences-sponsored University of California San Diego Institutional Research and Academic Career Development Award (NIH/IRACDA K12 GM068524). A.D. and K.O. were supported by the Ludwig Institute for Cancer Research, which also provided them with research funding used to support this work. We are grateful to Andrew Chisholm for his advice in the early phases of this project, Ronald Biggs for contributions to this project after the initial method development phase, and Dave Jenkins and Andy Shiau for support and access to the Small Molecule Discovery group’s high-content imaging system.
Materials
| Aspirator Tube Assembly | Drummond Scientific | 2-000-000 | |
| Calibrated Pipette (25mL) | Drummond Scientific | 2-000-025 | |
| Cell Voyager Software | Yokogawa Electric Corp | Included with CV1000 | |
| Conical Tube (15 mL ) | USA Scientific | 1475-0501 | |
| CV1000 Microscope | Yokogawa Electric Corp | CV1000 | |
| Depression slide (3-well) | Erie Scientific | 1520-006 | |
| Dissection Microscope | Nikon | SMZ-645 | |
| Eppendorf Centrifuge 5810R | Eppendorf | 5811 07336 | |
| ImageJ/FIJI | Open Source | https://imagej.net/Fiji | |
| M9 Buffer | Lab Prepared | https://openwetware.org/wiki/M9_salts | |
| Microcentrifuge Tube (1.5 mLl) | USA Scientific | 1615-5500 | |
| Microseal F-foil Seal | Bio-Rad | MSF1001 | |
| NGM Plates | Lab Prepared | http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html#d0e214 | |
| Scalpel #15 | Bard Parker | REF 371615 | |
| Sensoplate Plus, 384 Well, F-bottom, Glass Bottom | Greiner Bio-One | 781855 | |
| Tetramisole Hydrochloride | Sigma Aldirch | T1512-10G | |
| Tweezers, Dumont #3 | Electron Microscopy Sciences | 0109-3-PO | |
| U-PlanApo objective (10× 0.4NA) | Olympus | 1-U2B823 | |
| U-PlanApo objective (60× 1.35 NA) | Olympus | 1-U2B832 |
参考文献
- Armenti, S. T., Nance, J. Adherens junctions in C. elegans embryonic morphogenesis. Sub-Cellular Biochemistry. 60, 279-299 (2012).
- Chisholm, A. D., Hsiao, T. I. The Caenorhabditis elegans epidermis as a model skin. I: development, patterning, and growth. Wiley Interdisciplinary Reviews Developmental Biology. 1 (6), 861-878 (2012).
- Jackson, B. M., Eisenmann, D. M. beta-catenin-dependent Wnt signaling in C. elegans: teaching an old dog a new trick. Cold Spring Harbor Perspectives In Biology. 4 (8), 007948 (2012).
- Lamkin, E. R., Heiman, M. G. Coordinated morphogenesis of neurons and glia. Current Opinion in Neurobiology. 47, 58-64 (2017).
- Loveless, T., Hardin, J. Cadherin complexity: recent insights into cadherin superfamily function in C. elegans. Current Opinion in Cell Biology. 24 (5), 695-701 (2012).
- Priess, J. R. Notch signaling in the C. elegans embryo. WormBook. , 1-16 (2005).
- Spickard, E. A., Joshi, P. M., Rothman, J. H. The multipotency-to-commitment transition in Caenorhabditis elegans-implications for reprogramming from cells to organs. FEBS Letters. 592 (6), 838-851 (2018).
- Vuong-Brender, T. T., Yang, X., Labouesse, M. C. elegans Embryonic Morphogenesis. Current Topics in Developmental Biology. 116, 597-616 (2016).
- Wang, J. T., Seydoux, G. Germ cell specification. Advances in Experimental Medicine and Biology. 757, 17-39 (2013).
- Wang, S., et al. A high-content imaging approach to profile C. elegans embryonic development. Development. 146 (7), (2019).
- Wang, S., Ochoa, S., Khaliullin, R. N., Gerson-Gurwitz, A., Hendel, J. M., Zhao, Z., Biggs, R., Chisholm, A. D., Desai, A., Oegema, K., Green, R. A. . Dryad Digital Repository. , (2019).
- Wang, S., Ochoa, S., Khaliullin, R., Gerson-Gurwitz, A., Hendel, J., Zhao, Z., Biggs, R., Chisholm, A., Desai, A., Oegema, K., Green, R. A. . Zenodo. , (2018).
- . Software to crop C. elegans embryos from multi-channel microscopy images Available from: https://github.com/renatkh/embryo_crop (2018)
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9 (7), 671-675 (2012).
- Chisholm, A. D., Hardin, J. Epidermal morphogenesis. WormBook. , 1-22 (2005).
- Sulston, J. E., Schierenberg, E., White, J. G., Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. 発生生物学. 100 (1), 64-119 (1983).
- Fakhouri, T. H., Stevenson, J., Chisholm, A. D., Mango, S. E. Dynamic chromatin organization during foregut development mediated by the organ selector gene PHA-4/FoxA. PLoS Genetics. 6 (8), (2010).
- Zhong, M., et al. Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genetics. 6 (2), 1000848 (2010).
- Schmitz, C., Kinge, P., Hutter, H. Axon guidance genes identified in a large-scale RNAi screen using the RNAi-hypersensitive Caenorhabditis elegans strain nre-1(hd20) lin-15b(hd126). Proceedings of the National Academy of Sciences, USA. 104 (3), 834-839 (2007).
- Canny, J. A computational approach to edge detection. IEEE transactions on pattern analysis and machine intelligence. 8 (6), 679-698 (1986).
- Levin, M., Hashimshony, T., Wagner, F., Yanai, I. Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Developmental Cell. 22 (5), 1101-1108 (2012).
- Packer, J. S., et al. A lineage-resolved molecular atlas of C. elegans embryogenesis at single cell resolution. BioRxiv. , (2019).
- Oegema, K., Hyman, A. A. Cell division. WormBook. , 1-40 (2006).
- Insley, P., Shaham, S. Automated C. elegans embryo alignments reveal brain neuropil position invariance despite lax cell body placement. PLoS One. 13 (3), 0194861 (2018).

