Expression and Purification of Nuclease-Free Oxygen Scavenger Protocatechuate 3,4-Dioxygenase
概要
Protocatechuate 3,4-dioxygenase (PCD) can enzymatically remove free diatomic oxygen from an aqueous system using its substrate protocatechuic acid (PCA). This protocol describes the expression, purification, and activity analysis of this oxygen scavenging enzyme.
Abstract
Single molecule (SM) microscopy is used in the study of dynamic molecular interactions of fluorophore labeled biomolecules in real time. However, fluorophores are prone to loss of signal via photobleaching by dissolved oxygen (O2). To prevent photobleaching and extend the fluorophore lifetime, oxygen scavenging systems (OSS) are employed to reduce O2. Commercially available OSS may be contaminated by nucleases that damage or degrade nucleic acids, confounding interpretation of experimental results. Here we detail a protocol for the expression and purification of highly active Pseudomonas putida protocatechuate-3,4-dioxygenase (PCD) with no detectable nuclease contamination. PCD can efficiently remove reactive O2 species by conversion of the substrate protocatechuic acid (PCA) to 3-carboxy-cis,cis-muconic acid. This method can be used in any aqueous system where O2 plays a detrimental role in data acquisition. This method is effective in producing highly active, nuclease free PCD in comparison with commercially available PCD.
Introduction
Single molecule (SM) biophysics is a rapidly growing field changing the way we look at biological phenomena. This field has the unique ability to link fundamental laws of physics and chemistry to biology. Fluorescence microscopy is one biophysical method that can achieve SM sensitivity. Fluorescence is used to detect biomolecules by linking them to small organic fluorophores or quantum dots1. These molecules can emit photons when excited by lasers before photobleaching irreversibly2. Photobleaching occurs when the fluorescent labels undergo chemical damage which destroys their ability to excite at the desired wavelength2,3. The presence of reactive oxygen species (ROS) in aqueous buffer are a primary cause of photobleaching2,4. Additionally, ROS can damage biomolecules and lead to erroneous observations in SM experiments5,6. To prevent oxidative damage, oxygen scavenging systems (OSS) can be used3,7,8. The glucose oxidase/catalase (GODCAT) system is efficient at removing oxygen8, but it produces potentially damaging peroxides as intermediates. These may be damaging to biomolecules of interest in SM studies.
Alternatively, protocatechuate 3,4 dioxygenase (PCD) will efficiently remove O2 from an aqueous solution using its substrate protocatechuic acid (PCA)7,9. PCD is a metalloenzyme that uses nonheme iron to coordinate PCA and catalyze the catechol ring opening reaction using dissolved O210. This one step reaction is shown to be an overall better OSS for improving fluorophore stability in SM experiments7. Unfortunately, many commercially available OSS enzymes, including PCD, contain contaminating nucleases11. These contaminants can lead to the damage of nucleic acid-based substrates used in SM experiments. This work will elucidate a chromatography-based purification protocol for the use of recombinant PCD in SM systems. PCD can be broadly applied to any experiment where ROS are damaging substrates needed for data acquisition.
Protocol
1. Induce PCD expression in E. coli
- Combine 1 μL pVP91A-pcaHG PCD expression plasmid (20 ng/μL, Figure 1A) and 20 μL of E.coli BL21 (20 μL commercially available cells, > 2 x 106 cfu/μg plasmid) in a tube. Flick the tube to mix. Place the tube on ice 5 min.
- Place transformation at 42 °C for 30 s. Then ice 2 min.
- Add 80 μL SOC media (super optimal broth with catabolite repression: 2.5 mM KCl, 10 mM NaCl, 2% tryptone, 0.5% yeast extract, 10 mM MgSO4, 10 mM MgCl2, 20 mM glucose). Shake at 225 revolutions per min (rpm) 37 °C for 1 h.
- Plate the transformation reaction on LB Amp Agar (1L Luria Broth agar: 10 g NaCl, 10 g bacto-tryptone, 5 g yeast extract, 15 g agar, 50 μg/mL ampicillin; 25 mL per 10 cm diameter petri dish).
- Incubate the plate, lid facing down, at 37 °C for 16-18 h.
- Inoculate 50 mL LB Amp (1L LB: 10 g bacto-tryptone, 10 g NaCl, 5 g yeast extract, 50 μg/mL ampicillin) in a 250 mL Erlenmeyer flask with one colony. Incubate at 37 °C for 16-18 h shaking at 225 rpm.
- Transfer 20 mL culture to a 4 L flask with 1 L LB Amp. Shake at 225 rpm at 37 °C.
- Every h measure the culture OD600 (optical density at 600 nm). As the culture OD600 nears 0.5, increase the frequency of measurements to every 15 min. The desired density of the culture is 0.5 OD600.
- Transfer the 4L flask to a bin of ice. Swirl the flask in the ice bath to reduce the culture temperature.
Note: The time on ice should be kept to a minimum so that the cells remain metabolically active. Ideally the cells will be on ice less than 10 min. - Successful induction of PCD can be observed by denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Harvest 1 mL of the uninduced culture in a tube. Spin the sample 1 min at 14,000 x g in a microfuge at ambient temperature. Decant the supernatant.
- Solubilize the pelleted cells in 150 μL PBS (phosphate buffered saline).
- Add an equal volume of 2X loading dye (1.2% SDS, 30% glycerol, 150 mM Tris-HCl, pH 6.8, 0.0018% bromophenol blue, 15% β-mercaptoethanol). Vortex the sample to mix thoroughly.
- Boil the sample for 3 min and transfer to ice. The sample can be stored in a -20 °C freezer for future analysis.
Note: PBS is commercially available with or without CaCl2 and MgCl2. Many laboratories will have PBS without CaCl2 and MgCl2 for cell culture methods. We have found no difference with PBS with or without CaCl2 and MgCl2.
- Transfer the 4 L flask to an incubator at 17 °C with 180 rpm shaking. Continue to monitor the OD600 every 20 min.
- At 0.7 OD600 add isopropyl-beta-D-thiogalactopyranoside (IPTG) to 0.5 mM final concentration (0.25 M stock solution) and 10 mg/L ammonium iron (II) sulfate hexahydrate (Fe(NH4)2(SO4)2, 10 mg/mL stock solution). PCD genes pcaH and pcaG are induced from the T5 promoter by the addition of IPTG (Figure 1A). Iron sulfate is bound by PCD and required for catalytic activity by coordinating oxygen during catechol opening.
- Shake the culture at 180 rpm at 17 °C for 18 h.
- Place the culture flask in ice as in 1.2. Harvest 1 mL of the induced cells for SDS-PAGE as in 1.3.
- Pour the bacterial culture to bottles appropriate for centrifugation. A 1 L culture may be centrifuged in 4 250 mL conical bottom bottles. Pellet the culture at 4 °C for 20 min at 3000 x g. Decant the supernatants. Dispose of bacterial liquid waste appropriately.
- Pipet to resuspend the pellets in 25 mL cold PBS (CaCl2 and MgCl2 are optional) per 1 L culture.
- Transfer the resuspension to 50 mL conical tubes (1 50 mL tube per 1 L culture). Pellet the cells at 3000 x g for 20 min at 4 °C. Decant the supernatant and dispose appropriately.
- Resuspend the cells in 10 mL of lysis buffer (300 mM NaCl, 50 mM Tris-HCl, pH 7.5, 20 mM imidazole, 10 % glycerol, 800 ng/mL Pepstatin, 1 μg/mL Leupeptin, and 87.1 μg/mL phenylmethylsulfonyl fluoride (PMSF)) by pipetting. Freeze the resuspension with liquid nitrogen in a Dewar flask. Store the sample tubes in a -80 °C freezer. We have purified PCD from pellets stored at -80 °C for one year with no apparent loss of activity.
- Compare the uninduced and induced cells by SDS-PAGE.
Note: We have had no difficulty with induction of PCD heterodimer. However, we recommend testing the induction before continuing with the purification in the event any reagent has expired unexpectedly.- Assemble plates for SDS-PAGE (dimensions: 7.3 cm x 8.3 cm x 0.75 mm thick). The stacking gel is 1.5 mL 6% polyacrylamide (6% acrylamide, 125 mM Tris-HCl, pH 6.8, 0.1% SDS, 0.1% ammonium persulfate, 0.001% TEMED). The resolving gel is 3.5 mL 12% polyacrylamide (12% acrylamide, 375 mM Tris-HCl, pH 8.8, 0.1% SDS, 0.1% ammonium persulfate, 0.001% TEMED). Insert a 10 well comb to the stacking layer.
- Load 10 μL of uninduced and induced bacterial samples. Load 4 μL of prestained molecular weight markers for proteins (Figure 1B).
- Electrophorese the gel at 16.5 V/cm approximately 1 h. The bromophenol blue should reach the bottom of the gel.
- Place the gel in Coomassie Blue stain (10% acetic acid, 40% methanol, 0.1% Coomassie Blue dye) in a plastic tub. The stain should completely immerse the gel. Stain at ambient temperature for 20 min. Gentle rotation during staining is optional.
- Replace the solution with destain (10% acetic acid, 40% methanol). Incubate at ambient temperature with optional gentle rotation until protein bands are readily visible.
- Replace the destain with deionized water. Among the bacterial proteins, the induced PCD subunits should be visible in the induced cells. Hexahistidine tagged PCD subunit pcaH has a molecular weight of 28.3 kDa and the pcaG subunit is 22.4 kDa. If the PCD subunits are not apparent, a new induction derived from a novel colony should be performed.
2. Nickel affinity chromatography purification of PCD
- Thaw on ice one 50 mL tube of induced cells. It may take 2-3 h to completely thaw the sample.
- Keeping the tube on ice, sonicate the sample at 30% amplitude for 1 min, cycling 1 s on and off. Use a tapered microtip (diameter 0.125 inches) sonicator. Maximum power is 400 W and frequency is 20 kHz and per 1 L culture pellet.
- Following sonication, add lysozyme to 0.2 mg/mL final concentration (10 mg/ml stock solution) and keep on ice for 30 min at 4 °C.
- Pour the bacterial lysate into a pre-chilled polycarbonate bottle (dimensions: 25 mm x 89 mm). The bottle should be compatible with a fixed angle ultracentrifuge rotor. Other tubes and/or rotors may be substituted, but the final gravitation force should be maintained.
- Centrifuge for 60 min at 120,000 x g at 4 °C. Cellular debris will form a pellet. The supernatant may appear yellow.
- The pellet may be included in subsequent SDS-PAGE analysis to determine the solubility of PCD (Figure 1B). Solubilize the pellet by vortexing in 10 mL PBS. Transfer 150 μL to a 1.5 mL tube. Prepare the sample for SDS-PAGE as in 1.3.
- Pour the supernatant to a cold 50 mL conical tube. Note the volume. Contamination of the supernatant with bacterial DNA may yield a viscous sample. The bacterial genomic DNA could block the column flow. The ultracentrifugation step 2.2 should be repeated to pellet the bacterial DNA. A pellet from this second spin may not be readily visible or may be transparent.
- Make 500 mL Ni Buffer A (300 mM NaCl, 50 mM Tris-HCl, pH 7.5, and 10% glycerol, 800 ng/mL Pepstatin, 1 μg/mL Leupeptin, and 87.1 μg/mL PMSF) and 500 mL Ni Buffer B (300 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10% glycerol, 800 ng/mL Pepstatin, 1 μg/mL Leupeptin, and 87.1 μg/mL PMSF, 250 mM imidazole, pH 8.0). Pass both Ni Buffers through 0.2 μm pore filters.
- The sample, buffers, and the FPLC (fast protein liquid chromatography) system are in a refrigerated room at 4 °C. Wash pump A with Ni Buffer A and pump B with Ni Buffer B. Wash the system with 20 mM imidazole (8% Ni Buffer B, 92% Ni Buffer A) until the UV and conductivity stabilize. We routinely flow buffers at 5 mL/min with a 1.0 MPa pressure limit. The flow rate and pressure limit should be determined by the specifications of the FPLC instrument used.
- Prepare a column with 1.5 mL nickel-charged resin (dimensions: 110 mm long x 5 mm). The resin binding capacity is 50 mg/mL and can tolerate 1 MPa pressure. A column may be poured and stored at 4 °C before purification.
Note: The size of the column may be proportionally increased to accommodate more than one 50 mL tube of induced cells if more protein is required. We prefer a fresh column for each preparation to ensure that no residual proteins contaminate our desired protein. However, nickel resins may be recycled according to manufacturer's instructions. - Attach the column of nickel-charged resin to the FPLC. Run 20 mL of 92% Ni Buffer A and 8% Ni Buffer B (20 mM imidazole) at 0.5 mL/min with a 0.5 MPa pressure limit through the column to equilibrate. In real time the FPLC should measure A280 (280 nm UV absorbance) as well as conductivity. If these values have not stabilized after 20 mL volume has passed through the column, flow the buffers until they have stabilized.
- Load the sample to the column (~10 mL) at 0.15 mL/min. Set the pressure limit to 0.5 MPa. Collect the flow through.
- Wash the column with 20 mL Ni Buffer at 20 mM imidazole (92% Ni Buffer A and 8% Ni Buffer B). Retain the wash in a 50 mL tube for analysis. Wash the column with 15 mL of 50% Ni Buffer A and 50% Ni Buffer B (125 mM imidazole). Collect the elution in 19 fractions of 0.8 mL volume. Wash the column with 15 mL 100% Ni Buffer B. Collect an additional 75 fractions of 0.2 mL. Some PCD heterodimer will elute in the 50% Ni Buffer B wash, but the majority of the heterodimer will elute in the 100% Ni Buffer B wash.
- Analyze collected fractions on 12% SDS-PAGE gels to confirm presence of PCD. Add equal volumes of 2X loading dye to the flow through, wash, and peak A280 fractions. Boil for 3 min. Transfer to ice. Pour two 12% SDS-PAGE gels (as in step 1.7). Repeat the gel method as described in 1.7.
3. Nuclease activity assay
- Based on the SDS-PAGE analysis, identify nickel affinity fractions that contain nearly pure PCD. Combine 5 μL chromatography fraction and 500 ng 3 kb supercoiled plasmid pXba+ in reaction buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 0.1 mM DTT) with a final volume of 50 μL.
- Incubate at 37 °C for 1 h.
- Include a negative control (no added protein) and a positive control (commercially available PCD). Stop the reaction with 10 μL stop solution (150 mM EDTA, pH 8.0, 0.6% SDS, 18% glycerol, 0.15% Orange G).
- Samples may be kept in a -20 °C freezer to be analyzed later. Any supercoiled plasmid may be used in a nuclease assay as long as the supercoiled and relaxed circle reaction products may be resolved by agarose gel electrophoresis.
- Pour a 120 mL 1% agarose gel in 1X TAE ethidium buffer (40 mM Tris-acetate, 1 mM EDTA, 0.5 µg/mL ethidium bromide) in a gel cast (dimensions: 15 x 10 cm). Use a 15 well comb (well dimensions: 5mm x 1.5 mm). When the gel has set, immerse it in 1X TAE ethidium buffer.
- Analyze 30 μL of the reactions by agarose gel. Electrophorese at 10 V/cm at ambient temperature for approximately 1 h. The Orange G dye front should be at the end of the gel.
- Use a fluorescence scanner to immediately image the ethidium bromide signal of the gel. If a 3 kb plasmid was used, the slowest band will be relaxed circles at ~3.5 kb, linear DNA will run at 3 kb, and supercoiled plasmid will have the fastest mobility at ~2 kb.
- Calculate the total pixel volume of each lane with image analysis software.
- Determine the pixel volume of the various DNA species, such as supercoiled, linear, and nicked circles. Use these values to determine the percentage of each DNA species. For example, increased presence of nicked circles associated with a fraction compared to the negative control indicates the presence of nuclease. The pixel volume of nicked circles in a lane is divided by the pixel value of total DNA. Determine a percentage by multiplying this number by 100.
- Combine fractions from the second elution peak that contain nearly pure PCD heterodimer based on SDS-PAGE analysis and have minimal to undetectable nuclease activity. Our typical pooled volume is ~2 mL.
- Load the sample to a centrifugal filter unit with a 10 kDa molecular weight cutoff. Centrifuge in a swinging bucket centrifuge at 4000 x g for 40 min at 4 °C. Alternatively a 35° fixed angle rotor may be used at 7500 x g for 20 min at 4 °C.
- Repeat the centrifugation until the final retentate volume is 100-200 μL.
- Invert the filter unit and recover the retentate by centrifugation at 1000 x g for 2 min at 4 °C.
4. Size exclusion chromatography purification of PCD
- Make 250 mL size exclusion chromatography (SEC) running buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10% glycerol, 0.1 mM EDTA, 800 ng/mL Pepstatin, 1 μg/mL Leupeptin, and 87.1 μg/mL PMSF). Pass the buffer through a 0.2 μm pore filter and store at 4 °C.
Note: Perform all steps in a refrigerated room at 4 °C. SEC purification is optional, but the protein should be stored in SEC running buffer. If SEC purification is omitted, the retentate collected in 3.6.2. should be dialyzed against 1 L of SEC buffer in 10 kDa MWCO (molecular weight cut off) dialysis tubing at 4 °C overnight. - Equilibrate a cross-linked agarose SEC (size exclusion chromatography) column (dimesions: 10 mm x 300 mm; 24 mL bed volume; 25 – 500 μL sample volume; 1.5 MPa pressure limit; 2 x 106 Da exclusion limit; 1 to 300 kDa separation) with SEC Running Buffer at 0.5 mL/min.
Note: If desired alternative size SEC columns may be used. - Load the concentrated fractions to a 200 μL volume injection loop. Load the sample at 0.5 mL/min to the column. SEC resolution increases with smaller load volume. Elute with 23 mL SEC running buffer and collect 94 fractions of 250 μL. The SEC chromatogram should resolve a single A280 peak that is the PCD heterodimer. We find that PCD elutes 8.9 mL. The elution timing will change with alternative SEC columns.
5. PCA oxidation and nuclease activity assays
- Reactions to assay both oxidation of PCA and nuclease activity are performed in a 96-well flat-bottom plate. Assemble reactions in a 96 well plate on ice in a 4 °C cold room to prevent premature catalysis. Combine in a final volume of 50 μL: 130 mM NaCl, 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 0.1 mM DTT, 5 mM PCA, 10 ng/mL supercoiled plasmid pXba+, and 10 μL of individual PCD SEC fractions.
Note: The PCD SEC fractions should be added last and immediately before analysis as the protein will begin catalysis at the time of addition. - PCD oxidation of PCA results in reduced absorbance of PCA at 290 nm (A290). Transfer the 96 well plate to the plate holder of a plate reader set to an internal temperature of 37 °C. Retract the plate holder into the instrument and measure A290 at 20 s intervals for 1 h. Have the instrument shake the plate 5 s before each reading.
- After 1 h, terminate the reactions by adding 10 μL stop solution (150 mM EDTA, pH 8.0, 0.6% SDS, 18% glycerol, 0.15% Orange G).
- Prepare, load, and run an agarose gel as in step 3.2.
- Image and analyze the agarose gel as in step 3.3.
- Select fractions with the most PCA oxidation activity and no observed nuclease contamination for long-term storage at -80 °C. Measure the A280.
- Calculate the total PCD concentration using the A280 and the extinction coefficient (ε280) of 734,700 M-1cm-1.
- Snap freeze individual fractions in liquid nitrogen. Store in a -80 °C freezer. Alternatively, combine active, nuclease-free fractions, aliquot, and freeze in the same way. Our typical yield is 1-2 mg PCD per L culture. Typical use in a SM experiment is 3 μg PCD. We have used PCD stored at -80 °C for up to 1 year with no decrease in activity.
Representative Results
Commercially available oxygen scavenger PCD is frequently contaminated with a DNA nuclease. Contaminating nuclease activity could lead to spurious results in fluorescent studies, particularly studies that analyze DNA or DNA interacting proteins.We have found that recombinant PCD, a heterodimer of hexahistidine tagged pcaH and pcaG, may be expressed in E. coli (Figure 1). The heterodimer is first purified by nickel affinity chromatography (Figure 2). PCD is eluted in 2 steps of imidazole concentrations. Chromatography fractions are analyzed by SDS-PAGE. Fractions of nearly pure PCD are concentrated and further purified by SEC (Figure 3). SEC fractions are individually analyzed for both PCA oxidation activity and nuclease activity (Figure 4). Fractions that displayed high oxidation activity and no apparent nuclease activity are assayed for protein concentration and kept in a -80 °C freezer for experimental use.
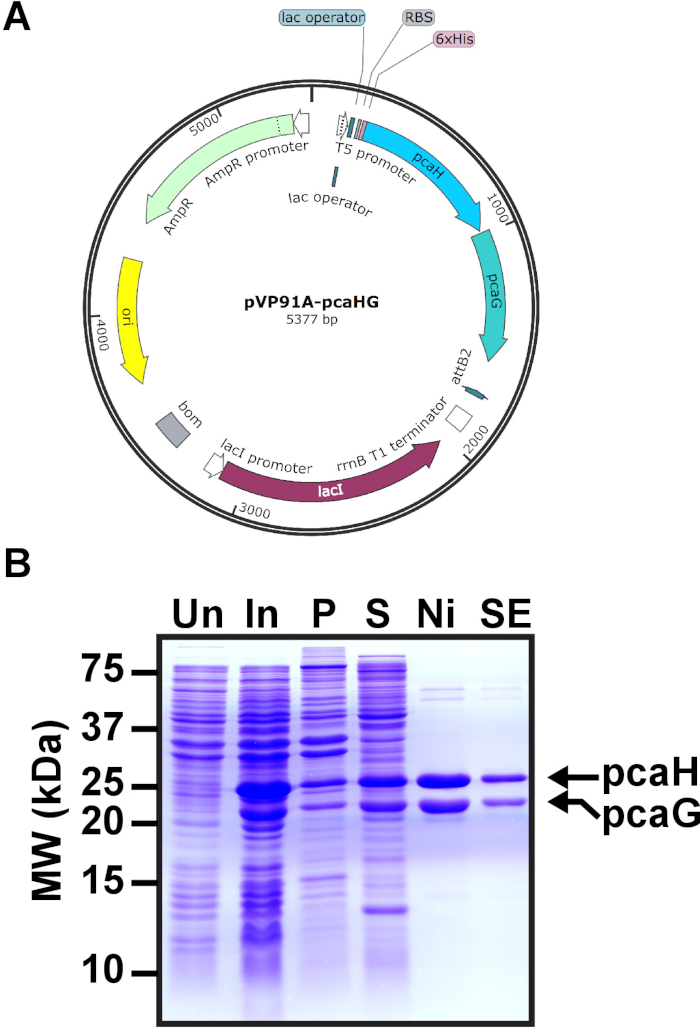
Figure 1: Induction of PCD in E. coli. (A) pVP91A-pcaHG is shown with the pcaG (α) and hexahistidine-tagged pcaH (β) PCD subunits. (B) Representative SDS-PAGE gel of PCD induction. Molecular weights are indicated on the left. The mobilities of 28.3 kDa hexahistidine-tagged pcaH and 22.4 kDa pcaG are on the right. Uninduced E. coli (Un), induced E. coli (In), the pellet following E. coli lysis and ultracentrifugation (P), the supernatant following ultracentrifugation to be loaded to a nickel column (S), representative fraction following nickel chromatography (Ni), and representative fraction following SEC (SE). This figure has been modified from a previous publication12. Please click here to view a larger version of this figure.
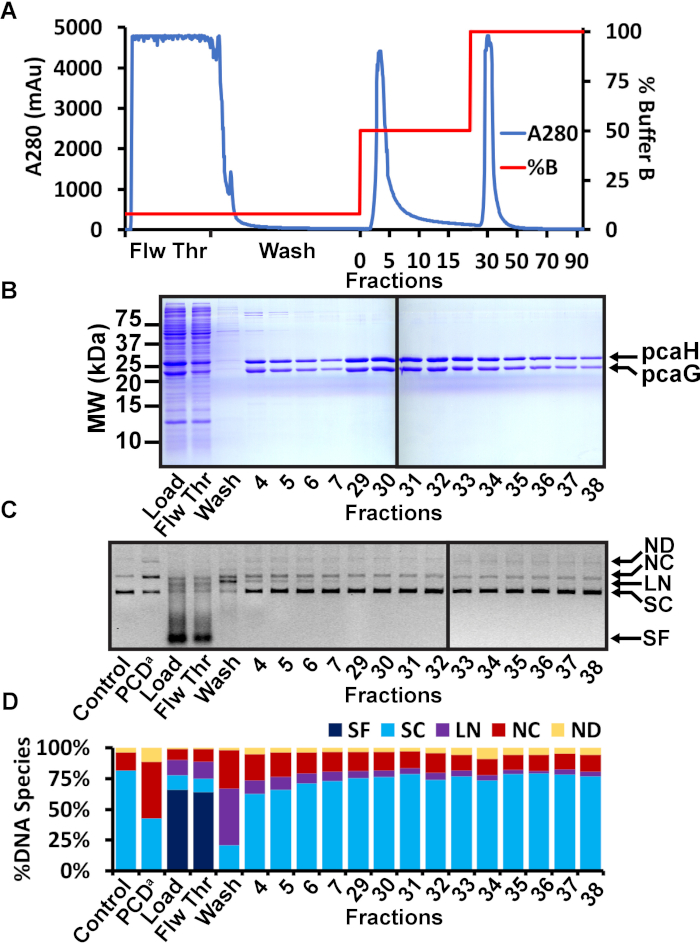
Figure 2: Nickel affinity chromatography purification of PCD. (A) Chromatogram of nickel affinity chromatography of PCD. The A280 is shown in blue and the percent concentration of Ni Buffer B is shown in red. The sample was loaded in a low 20 mM imidazole concentration. The flowthrough (Flw Thr) shows the soluble bacterial proteins that did not bind to the nickel resin. The column was washed with 20 mL of 20 mM imidazole buffer. A second 15 mL wash was performed with 125 mM imidazole. Elution of PCD was performed with 250 mM imidazole. Some PCD eluted in the presence of 125 mM imidazole, but the majority of the protein eluted in 250 mM imidazole. (B) Representative SDS-PAGE analysis of nickel affinity fractions. The load, flowthrough (Flw Thr), and first wash showed the successful induction of PCD, the soluble bacterial proteins that did not bind the nickel resin, and the minimal proteins observed during the first wash, respectively. Several fractions throughout the second wash and elution steps are shown. Fractions from the second wash included PCD protein but also displayed detectable higher molecular weight contaminants. Fractions from the elution step appeared to be free of contaminants. Molecular weights are shown on the left. Mobilities of pcaH and pcaG are shown on the right. (C) Agarose gel of nuclease assay. The nickel affinity column load, flowthrough, wash, and multiple fractions were tested for nuclease activity. A negative control (control) is the plasmid without added protein. A positive control (PCDa) is a commercially available PCD known to be contaminated with a DNA nuclease. DNA species are indicated on the right as small fragments (SF), supercoiled (SC), linear (LN), nicked circle (NC), and nicked dimer (ND). (D) Quantitation of the various DNA species observed in the agarose gel nuclease assay. The total pixel volume of each lane was measured. The pixel volume of each DNA species was determined and expressed as a percentage of the total pixel volume in the lane. The negative control was 81.7% supercoiled with 14.4% nicked circles. The positive control displayed a significant increase of 46.0% nicked circles. The load and flowthrough contained bacterial nucleases that converted the plasmid and contaminating bacteria DNA to small fragments. The first wash at 20 mM imidazole also appeared to contain significant nuclease activity, resulting in linear and nicked circles. Fractions 4-7 from the second wash at 125 mM imidazole also displayed significant nuclease activity (particularly, fractions 4 and 5 that generated observed linearized plasmid). Fractions 29-38 from the elution step appeared more similar to the negative control. In this example, fractions 29-38 were chosen to be combined, concentrated, and further purified by SEC. This figure has been modified from a previous publication12. Please click here to view a larger version of this figure.
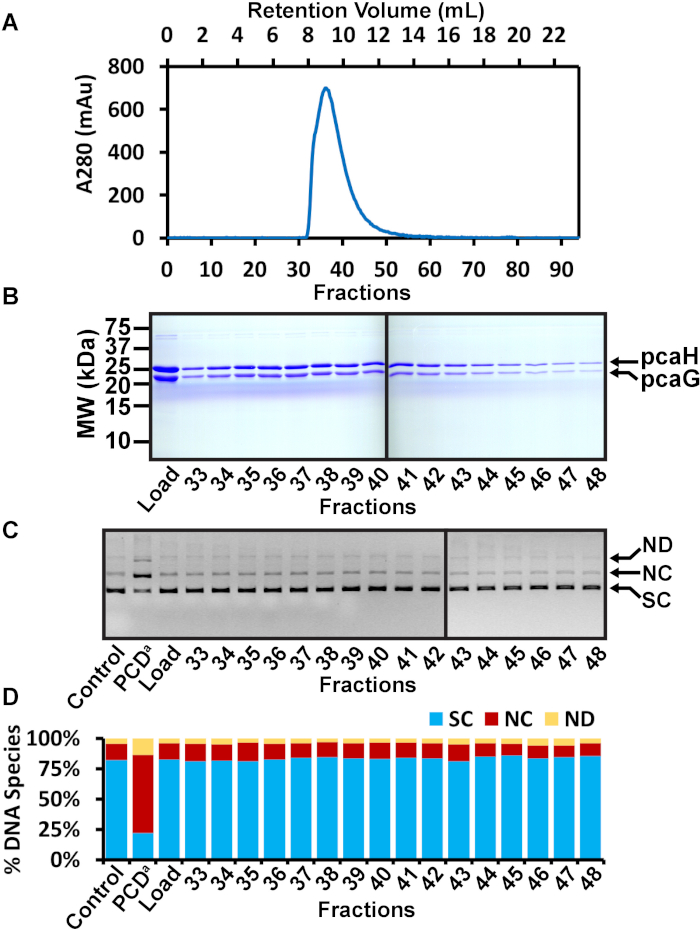
Figure 3: SEC purification of PCD. (A) Chromatogram of SEC of PCD fractions following nickel affinity chromatography. The A280 is shown in blue and elution fractions are indicated. PCD eluted from SEC as a single apparent peak. (B) Representative SDS-PAGE analysis of SEC fractions 33-48. The load is the concentrated PCD following nickel affinity purification. Fractions 33-48 span the apparent SEC peak. No detectable contaminants were observed. (C) Agarose gel of nuclease assay. The SEC load and multiple fractions were tested for nuclease activity. A negative control (control) is the plasmid without added protein. A positive control (PCDa) is a commercially available PCD known to be contaminated with a DNA nuclease. DNA species are indicated on the left as supercoiled (SC), nicked circle (NC), and nicked dimer (ND). (D) Quantitation of the various DNA species observed in the agarose gel nuclease assay. The total pixel volume of each lane was measured. The pixel volume of each DNA species was determined and expressed as a percentage of the total pixel volume in the lane. The negative control was 82.1% supercoiled with only 13.7% nicked circles. The positive control displayed a significant increase of 64.8% nicked circles. The SEC load displayed no apparent nuclease activity due to judicious choice of fractions from the nickel affinity purification. Similarly, fractions 33-48 appeared similar to the negative control. For example, fraction 36 was 82.5% supercoiled and 13.2% nicked circle. In this example, fractions 36 and 37 were chosen to be quantified, frozen, and kept in a -80 °C freezer for future experimental use. This figure has been modified from a previous publication12. Please click here to view a larger version of this figure.
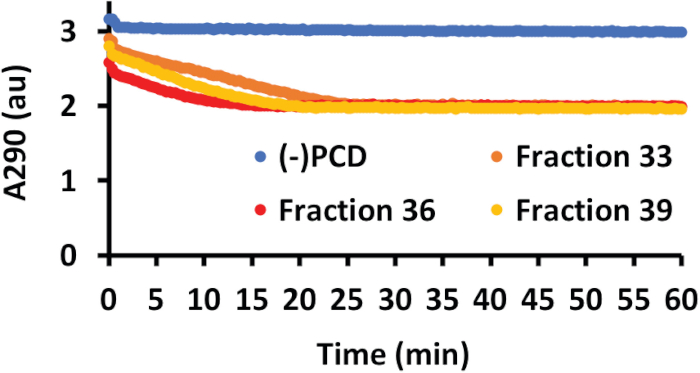
Figure 4: PCA oxidation and nuclease activity of PCD SEC fractions. PCA oxidation was measured by A290. As PCD oxidized the PCA molecule, the A290 decreased. PCA oxidation was measured every 20 s for 1 h. A negative control with no added PCD fraction (blue line) showed no change in A290, indicating the PCA molecule was stable. Data from three representative SEC fractions (36 in red, 33 in orange, 39 in yellow) show that purified PCD reduced the A290, indicating oxidation of PCA. This figure has been modified from a previous publication12. Please click here to view a larger version of this figure.
Discussion
Oxygen scavenging systems are commonly included in single molecule fluorescence microscopy to reduce photobleaching3,7,8. These microscopy techniques are often used to observe nucleic acids or protein interactions with nucleic acids1,13,14. Contamination of OSSs with nucleases may lead to spurious results.
Commercially available OSSs, including GODCAT and PCD, have been shown to include significant nuclease contamination11. It is possible to purchase PCD and employ SEC to remove the nuclease contaminant11. However, the price of commercially available PCD from one vendor increased 5 fold following the publication of that method. This method generates a highly active, nuclease free PCD heterodimer and can conceivably be performed within 1 week. In our experience, the amount of PCD generated by a 1 L culture (1-2 mg) is sufficient for one year of experiments (3 μg/experiment) in a productive laboratory with 2 fluorescent imaging systems.
Induction efficiency is key to the success of this method. If the PCD heterodimer is not efficiently induced and apparent by SDS-PAGE, the purification will be unsuccessful. Two alternative strategies may be tried. First, attempt induction from a different colony on the E. coli transformation plate. Second, we have had previous success with BL21, but an alternative E. coli strain for expression, such as BL21 pLysS, may be tried. Success of the PCA oxidation assay relies on minimal exposure of the substrate to the PCD before starting the assay. It is highly recommended to assemble the 96 well plate reactions on ice in a cold room and add the protein sample immediately before loading the plate to the reader.
Nickel affinity chromatography may be sufficient to identify fractions of pure PCD with no contaminating nuclease activity. In this case, it is possible to eliminate the SEC purification. However, the nickel chromatography fractions should be combined and dialyzed overnight at 4 °C in SEC running buffer. The glycerol present in the SEC running buffer is important for storage at -80 °C.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH GM121284 and AI126742 to KEY.
Materials
| 2-Mercaptoethanol | Sigma-Aldrich | M3148 | βME |
| 30% acrylamide and bis-acrylamide solution, 29:1 | Bio-Rad | 161-0156 | |
| Acetic acid, Glacial Certified ACS | Fisherl Chemical | A38C-212 | |
| Agar, Granulated | BD Biosciences | DF0145-17-0 | |
| AKTA FPLC System | GE Healthcare Life Sciences | AKTA Purifier: Box-900, pH/C-900, UV-900, P-900, and Frac-920 | |
| Amicon Ultra-2 Centrifugal Filter Unit | EMD Millipore | UFC201024 | 10 kDa MWCO |
| Ammonium iron(II) sulfate hexahydrate | Sigma | F-2262 | |
| Ammonium Persulfate (APS) Tablets | Amresco | K833-100TABS | |
| Ampicillin | Amresco | 0339-25G | |
| Bacto Tryptone | BD Biosciences | DF0123173 | |
| BD Bacto Dehydrated Culture Media Additive: Bottle Yeast Extract | VWR | 90004-092 | |
| BIS-TRIS propane,>=99.0% (titration) | Sigma-Aldrich | B6755-500G | |
| Bromophenol Blue | Sigma-Aldrich | B0126-25G | |
| Coomassie Brilliant Blue | Amresco | 0472-50G | |
| Costar 96–Well Flat–Bottom EIA Plate | Bio-Rad | 2240096EDU | |
| DTT | P212121 | SV-DTT | |
| Dulbecco's Phosphate Buffered Saline 500ML | Sigma-Aldrich | D8537-500ML | PBS |
| Ethidium bromide | Thermo Fisher Scientific | BP1302 | |
| Glycerol | Fisher Scientific | G37-20 | |
| Granulated LB Broth Miller | EMD Biosciences | 1.10285.0500 | |
| Hi-Res Standard Agarose | AGTC Bioproducts | AG500D1 | |
| Imidazole | Sigma-Aldrich | I0250-250G | |
| IPTG | Goldbio | I2481C25 | |
| Leupeptin | Roche | 11017128001 | |
| Lysozyme from Chicken Egg White | Sigma-Aldrich | L6876-1G | |
| Magnesium Chloride Hexahydrate | Amresco | 0288-1KG | |
| Microvolume Spectrophotometer, with cuvet capability | Thermo Fisher | ND-2000C | |
| NaCl | P212121 | RP-S23020 | |
| Ni-NTA Superflow (100 ml) | Qiagen | 30430 | |
| Novagen BL21 Competent Cells | EMD Millipore | 69-449-3 | SOC media included |
| Orange G | Fisher Scientific | 0-267 | |
| Pepstatin | Gold Biotechnology | P-020-25 | |
| PMSF | Amresco | 0754-25G | |
| Protocatechuic acid | Fisher Scientific | ICN15642110 | PCA |
| Sodium dodecyl sulfate | P212121 | CI-00270-1KG | |
| SpectraMax M2 Microplate Reader | Molecular Devises | ||
| Sterile Disposable Filter Units with PES Membrane > 250mL | Thermo Fisher Scientific | 09-741-04 | |
| Sterile Disposable Filter Units with PES Membrane > 500mL | Thermo Fisher Scientific | 09-741-02 | |
| Superose 12 10/300 GL | GE Healthcare Life Sciences | 17517301 | |
| TEMED | Amresco | 0761-25ML | |
| Tris Ultra Pure | Gojira Fine Chemicals | UTS1003 | |
| Typhoon 9410 variable mode fluorescent imager | GE Healthcare Life Sciences | ||
| UltraPure EDTA | Invitrogen/Gibco | 15575 | |
| ZnCl2 | Sigma-Aldrich | 208086 |
参考文献
- Shera, E. B., Seitzinger, N. K., Davis, L. M., Keller, R. A., Soper, S. A. Detection of single fluorescent molecules. Chemical Physics Letters. 174 (6), 553-557 (1990).
- Zheng, Q., Jockusch, S., Zhou, Z., Blanchard, S. C. The contribution of reactive oxygen species to the photobleaching of organic fluorophores. Photochemistry and Photobiology. 90 (2), 448-454 (2014).
- Ha, T., Tinnefeld, P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annual Review of Physical Chemistry. 63, 595-617 (2012).
- Dixit, R., Cyr, R. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. The Plant Journal: for Cell and Molecular Biology. 36 (2), 280-290 (2003).
- Davies, M. J. Reactive species formed on proteins exposed to singlet oxygen. Photochemical & Photobiological Sciences. 3 (1), 17-25 (2004).
- Sies, H., Menck, C. F. Singlet oxygen induced DNA damage. Mutation Research. 275 (3-6), 367-375 (1992).
- Aitken, C. E., Marshall, R. A., Puglisi, J. D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophysical Journal. 94 (5), 1826-1835 (2008).
- Harada, Y., Sakurada, K., Aoki, T., Thomas, D. D., Yanagida, T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. Journal of Molecular Biology. 216 (1), 49-68 (1990).
- Shi, X., Lim, J., Ha, T. Acidification of the oxygen scavenging system in single-molecule fluorescence studies: in situ sensing with a ratiometric dual-emission probe. Analytical Chemistry. 82 (14), 6132-6138 (2010).
- Brown, C. K., Vetting, M. W., Earhart, C. A., Ohlendorf, D. H. Biophysical analyses of designed and selected mutants of protocatechuate 3,4-dioxygenase1. Annual Review of Microbiology. 58, 555-585 (2004).
- Senavirathne, G., et al. Widespread nuclease contamination in commonly used oxygen-scavenging systems. Nature Methods. 12 (10), 901-902 (2015).
- Senavirathne, G., Lopez, M. A., Messer, R., Fishel, R., Yoder, K. E. Expression and purification of nuclease-free protocatechuate 3,4-dioxygenase for prolonged single-molecule fluorescence imaging. Analytical Biochemistry. 556, 78-84 (2018).
- Jones, N. D., et al. Retroviral intasomes search for a target DNA by 1D diffusion which rarely results in integration. Nature Communications. 7, 11409 (2016).
- Liu, J., et al. Cascading MutS and MutL sliding clamps control DNA diffusion to activate mismatch repair. Nature. 539 (7630), 583-587 (2016).

