Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus
概要
Lung ultrasound is a noninvasive and valuable tool for bedside evaluation of neonatal lung diseases. However, a relative lack of reference standards, protocols and guidelines may limit its application. Here, we aim to develop a standardized neonatal lung ultrasound diagnostic protocol to be used in clinical decision-making.
Abstract
Ultrasound is a safe bedside imaging tool that obviates the use of ionizing radiation diagnostic procedures. Due to its convenience, the lung ultrasound has received increasing attention from neonatal physicians. Nevertheless, clear reference standards and guideline limits are needed for accurate application of this diagnostic modality. This document aims to summarize expert opinions and to provide precise guidance to help facilitate the use of the lung ultrasound in the diagnosis of neonatal lung diseases.
Introduction
The chest x-ray (CXR) and/or chest computerized tomography (chest CT) are the main imaging tools in the diagnosis of lung diseases. For a long time, the lung ultrasound (LUS) was considered a "forbidden zone” in the diagnosis of lung diseases since ultrasonic waves are totally reflected when encountering air. However, by utilizing ultrasonic artifacts formed by different pathological changes in adults, children and newborn infants1,2,3,4,5, this "forbidden zone" has been contested and point-of-care lung ultrasound (POC-LUS) has been successfully used for the diagnosis of lung diseases. Some authors have recommended POC-LUS as a preferred imaging modality in evaluation of lung diseases due to its greater accuracy, reliability, ease of performance and lack of potential adverse effects (i.e., radiation)5,6,7. In some neonatal intensive care units (NICUs), POC-LUS has replaced CXR and become the first-line approach used for the diagnosis and differential diagnosis of various neonatal lung diseases5,6,7,8,9.
Nonetheless, the use of POC-LUS remains limited due to the lack of operating protocols, diagnostic standards and guidelines. To promote proper utilization of POC-LUS in the neonatal field, the Division of Perinatology of the Society of Chinese Pediatrics and the Division of Neonatal Ultrasound Society of the Chinese Neonatologist Association in combination with the Chinese College of Critical Ultrasound have organized an international expert panel to review the latest publications on neonatal LUS. The panel summarized these expert-opinions and developed the present LUS protocols and guidelines for its use. The main objective is to popularize the application of POC-LUS in NICUs is reducing the number of CXR and thus avoiding the potential radiation-induced harmful effects. As a real-time imaging technique, LUS is user-friendly, easy to learn, and easy to replicate with appropriate training.
Patients and timing of the LUS examination
Indications for the initial POC-LUS exam include: (i) a neonate admitted for respiratory distress (ii) prenatal suspicion of lung lesions, and (iii) a neonate with a sudden deterioration of respiratory status.
Indications for a follow-up POC-LUS exam include: (i) helping to guide respiratory support (In experienced hands, ultrasonography-assisted weaning of mechanical ventilation may significantly shorten the duration of mechanical ventilation and reduce extubation failure.); (ii) helping to guide changes in the level of respiratory support after surfactant delivery as well as to determine the need for a repeat surfactant treatment; (iii) monitoring the progress of respiratory illness when needed; (iv) following up the changes in the lung volume or the degree of atelectasis in the post-bronchoalveolar lavage period (i.e., for infants with meconium aspiration syndrome, severe pneumonia, or atelectasis) as well as improving visualization of the therapeutic effects of thoracentesis (i.e. pleural effusion or pneumothorax)10,11.
Lung Ultrasonography Terminology
Pleural line and lung sliding12,13: A pleural line is a hyperechoic reflection formed by the difference in acoustic impedance between the pleural-lung surface interface. It appears as a smooth, regular and relatively straight hyperechoic line (Supplemental Figure 1). Blurring, irregularities, interruption of continuity or absence of the pleural line indicates abnormalities. In a real-time ultrasound, the pleural line moves in a to- and fro- pattern, synchronized with respiratory movement. This kind of movement is called lung sliding (Video 1). The absence of lung sliding is always pathologic.
A-line12,13: An A-line is a type of reverberation artifact caused by multiple reflections of the pleura when the probe is perpendicular to the ribs for scanning. A-lines are situated below the pleural line and present as a series of smooth, clear, regular and equidistant hyperechoic parallel lines. The echoes of the A-lines gradually diminish as they move deeper into the lung field where they ultimately disappear (Supplemental Figure 2).
B-line, confluent B-line and alveolar-interstitial syndrome13,14,15: Based on current literature and our clinical experiences in the field of neonatal lung diseases, we have defined these terms as follows: A single B-line is a type of linear hyperechoic reflection of an artifact caused by an ultrasound wave encountering the alveolar gas-liquid interface. B-lines arise from and are roughly vertical to the pleural line. They spread downwards to the edge of the screen without fading and move in synchrony with lung sliding. A confluent B-line is defined as the entire intercostal space filled with B-lines (B-line fusion, reflecting B-lines that are difficult to distinguish and count) between two acoustic shadows of the ribs. Alveolar-interstitial syndrome (AIS) is defined as two or more sequential intercostal spaces with confluent B-lines in any scanning area (Supplemental Figure 3).
Compact B-lines and white lung15,16: When the probe is used to scan perpendicular to the ribs, the presence of concentrated B-lines may cause the acoustic shadow of the ribs to disappear within the entire scanning zone. This type of B-line is called a compact B-line. A white lung is present when each scanning zone on both sides of the lung presents as compact B-lines. Compact B-lines and a white lung are manifestations of severe pulmonary edema (Supplemental Figure 4).
Lung consolidation and shred sign17,18: On LUS, lung fields may have a tissue-like density (lung tissue ‘hepatization’), which usually represents lung consolidation. Lung consolidation may be accompanied by air bronchograms, fluid bronchograms, or even dynamic air bronchograms in the most severe cases (Video 2). When the boundary between the consolidated lung tissue and the aerated lung tissue is unclear, the hyperechoic ultrasonic signs formed between the two areas are called shred signs (Supplemental Figure 5).
Lung pulse19: If lung consolidation is sufficiently large and near the edges of the heart, the consolidated lung may appear to be pulsating synchronized with the heartbeat when observed with real-time ultrasound. This sign is called the lung pulse (Video 3).
Lung point13,18,20: Under real-time ultrasound, the appearance of an alternate area where lung sliding is present and then absent is called a lung point. The lung point is a specific sign of a pneumothorax and can accurately locate the position of the gas boundary when a mild-moderate pneumothorax is present (Supplemental Figure 6).
Double lung point21: Due to the differences in the severities and/or the natures of the lesions in different area of the lungs, a clear difference between the upper and lower lung fields could be found with perpendicular scans, which forms a sharp cut-off point between the upper and lower lung field known as a double point (Supplemental Figure 7).
Sandy beach sign and stratosphere sign20,21,22: Under M-mode ultrasonography, a series of wavy-line echo above the pleural line and the uniform granular dot echo (generated by the lung sliding) below the pleural line can together form a beach-like sign known as a sandy beach sign or seashore sign. When lung sliding disappears, the granular dot echoes are replaced by a series of horizontal parallel lines. This kind of ultrasonic sign is known as a stratosphere sign or barcode sign (Supplemental Figure 8).
Protocol
This work was approved by the research ethics committee of Beijing Chaoyang District Committee of Science & Technology and the ethics committee of Beijing Chaoyang District Maternal and child Healthcare Hospital, and that the protocol follows the guidelines of the hospital’s human research ethics committee.
1. Ultrasound exam preparation
- Probe selection
- Select a high-frequency linear probe (≥9.0 MHz) for POC-LUS to ensure high resolution.
NOTE: A higher frequency linear probe is used to assure higher resolution. For infants with a lower gestational age or lower birth weight, a higher frequency probe is required. When the penetration is not enough, decrease the frequency or change to a lower frequency liner probe. If no suitable linear probe is available, consider using a high-frequency (≥8.0 MHz) convex array probe.
- Select a high-frequency linear probe (≥9.0 MHz) for POC-LUS to ensure high resolution.
- Probe disinfection
- Disinfect the probe before and after patient examination to avoid nosocomial infection and cross-contamination.
NOTE: The easiest, most convenient and effective disinfecting method is the use of special disinfection wipes. Alternatively, powderless gloves or probe covers can also be considered.
- Disinfect the probe before and after patient examination to avoid nosocomial infection and cross-contamination.
- Preset selection
- Choose a LUS preset.
- Optimize the image for lung scanning if there is no LUS preset.
- Select one of Small Parts presets.
- Modify the parameters to perform lung scanning. Adjust the Depth button to make it 4-5 cm.
- Press the Focus Zone button to have 1-2 focuses and adjust the focus position near the level of pleural line. Turn on the SRI (Speckle Reduction Imaging) button and select level 2-3 to reduce the speckle noise.
- Turn on the CRI button (Crossbeam) and select level 2 to improve contrast resolution. Activate the Harmonics to improve the signal-noise ratio or use the fundamental frequency for sharper A-lines or B-lines.
- Ultrasound gel application
- Warm up the gel.
- Apply a layer of gel on the transducer. Make sure to avoid air bubbles between the transducer and the skin surface.
2. Infant positioning
- Keep the infant in a quiet state.
- Swaddle the infant exposing only the area to be examined.
- Place the infant in the supine, prone or side position before and during the process of examination.
NOTE: In general, we do not recommend using sedatives while pacifier use is encouraged. Supine positioning is convenient for scanning of the anterior and lateral chest. Prone or side positioning is convenient for scanning of the back and lateral chest.
3. Lung partitioning
- Six-region method
- Divide each lung into three regions: anterior, lateral and posterior lung area. To do this, use the anterior axillary line and the posterior axillary line as boundaries. Divide both lungs into a total of six regions.
- Twelve-region method
- By using the line that connects the nipples, divide each lung into upper and lower lung fields, resulting in a total of 12 regions on both sides of the lungs.
NOTE: Carefully scan the entire lung fields. Each of the 6 or 12 areas should be scanned separately to ensure comprehensive coverage and to minimize the possibility of missing existing lung lesions.
- By using the line that connects the nipples, divide each lung into upper and lower lung fields, resulting in a total of 12 regions on both sides of the lungs.
4. Scan mode selection
- B-mode ultrasound
- Press the 2D button on the user interface to start B-mode scanning.
NOTE: B-mode scanning is the most important and the most commonly used mode in obtaining LUS images. The majority of lung diseases can be diagnosed with B-mode scanning.
- Press the 2D button on the user interface to start B-mode scanning.
- M-mode ultrasound
- Press the M button on the user interface to start M mode scanning if needed.
NOTE: M-mode ultrasound is helpful for further confirmation of the possibility of pneumothorax.
- Press the M button on the user interface to start M mode scanning if needed.
- Color or power Doppler ultrasound
- Press the C button or PD button on the user interface to start the color or power Doppler examination if needed.
NOTE: Doppler ultrasound is used occasionally to assess the blood flow in the large area of lung consolidations or to distinguish the bronchi from blood vessels.
- Press the C button or PD button on the user interface to start the color or power Doppler examination if needed.
5. Scanning methods
- Perpendicular scanning
- Place the transducer perpendicular to the ribs and slide it from the midline to the lateral side along the wide axis to perform the perpendicular scanning.
- After initial area of the lung is scanned, move the transducer from up to down and scan the remaining areas until all the lung fields are examined.
NOTE: Perpendicular scanning is the most important scanning method. Keeping the transducer perpendicular to the ribs is the key to obtaining accurate and reliable results.
- Parallel scanning
- Rotate the transducer 90° after finishing the perpendicular scanning. Keep the transducer parallel to the ribs and slide it along the narrow axis to realize the parallel scanning.
- After the initial area of the lung is scanned, move the transducer from up to down to scan the remaining areas until all the lung fields are examined.
- Transdiaphragmatic scanning
- Place the transducer below the xiphoid and angle the transducer from side to side to scan the diaphragm and the bottom of lungs via the liver as the acoustic window.
NOTE: Increase the depth and turn on virtual convex scanning to expand the far field area if needed.
- Place the transducer below the xiphoid and angle the transducer from side to side to scan the diaphragm and the bottom of lungs via the liver as the acoustic window.
Representative Results
The main purpose of this protocol and guideline is to instruct users on how to use LUS to diagnose and differentiate common neonatal lung diseases. These include respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), pneumonia, meconium aspiration syndrome (MAS), pulmonary hemorrhage, pulmonary atelectasis and pneumothorax, etc. Thus, the normal neonatal LUS characteristics and the LUS diagnostic criteria for different lung diseases are described in detail.
Normal Neonatal Lung Ultrasound
The neonatal normal lung field appears hypoechoic on a B-mode ultrasound. Pleural lines and A-lines are smooth, regular and straight. As mentioned previously, A-lines are hyperechoic, arranged in parallel and equidistant from one each other, which together form a kind of bamboo-like appearence known as the bamboo sign. A-line echoes gradually diminish until they disappear from the shallow to the deep part of the lung fields. There may not be any B-lines (three to seven days after birth) or just a few B-lines (within three to seven days after birth) in the lung fields. However, there is no AIS, pleural effusion or lung consolidation. Lung sliding is detectable by real-time ultrasound, whereas in M-mode imaging, a linear pattern appears in tissues superficial to the pleural line, and a grainy or sandy pattern appears below the pleural line, creating the seashore sign (Figure 1)23,24.
LUS Characteristics and Diagnostic Criteria for Lung Diseases of the Newborn Infants
Respiratory distress syndrome (RDS) of the newborn
RDS refers to a lung disease where main clinical manifestations are tachypnea, retractions, grunting and cyanosis. It presents immediately after birth. RDS is caused by a primary or secondary deficiency of pulmonary surfactant in preterm and term neonates respectively. Lack of surfactant causes development of pulmonary atelectasis and low lung volumes25,26,27. Currently, the diagnosis of RDS is based on history, clinical manifestations and CXR findings. However, RDS can also be diagnosed easily and accurately by LUS. A meta-analysis that included 673 newborn infants with RDS showed that the sensitivity and specificity of LUS in diagnosing RDS was 99% and 96%, respectively28.
LUS diagnosis of RDS is based on the following findings16,28,29,30,31,32,33,34. (i) Lung consolidations accompanied by air-bronchograms are the most important LUS manifestation of RDS, which is characterized by the following: (a) Consolidations are most often observed in the posterior parts of the lungs. The degree of consolidation is related to the severity of the disease. (b) Consolidations are limited only to the region beneath the pleura in mild RDS patients. Conversely, the areas of consolidation may extend to deeper parts of the lung fields in more severe RDS. (c) Usually, consolidations are visible in different lung fields bilaterally. Nevertheless, they may be limited to certain intercostal spaces on one side of the lung. Consolidated areas show an uneven hypoechoic quality and the boundary with surrounding lung tissue is clear and easy to distinguish. (d) Air-bronchograms show dense, speckled or snowflake-like shapes. (ii) The pleural line is abnormal, and the A-lines disappear. (iii) The non-consolidated zones may appear as AIS. (iv) 15% to 20% of patients may have different degrees of unilateral or bilateral pleural effusion.
In addition, changes in pulmonary status can be efficiently followed-up by LUS. Improvements in LUS findings are often first observed in anterior lung areas because these areas are non-dependent and better ventilated. Transition from consolidation to aggregation-induced emission (AIE), AIE to interstitial edema (IE), and IE to a normal LUS pattern or vice-versa can be seen. This LUS quality allows for estimation of the surfactant replacement therapy effect (Figure 2).
Transient tachypnea of the newborn (TTN)
TTN is also known as ‘wet lung’ of the newborn. It is one of the most common respiratory diseases in newborn infants. TTN is self-limited with most patients recovering within 24-72 hours without any special intervention. Rarely, it can lead to severe respiratory distress, hypoxemia, pneumothorax or even death35,36. TTN is often underdiagnosed, especially among premature infants. It has been reported that 62% to 77% of infants who were clinically diagnosed with RDS actually had TTN according to the traditional diagnostic criteria36,37. LUS can eliminate such misdiagnoses since TTN can be easily differentiated from RDS and other lung diseases by LUS.
The main characteristic of TTN is lung edema without lung consolidations, and it is diagnosed based on the following findings21,30,31,38,39. (i) Mild TTN mainly manifests as AIS and a double lung point. Severe TTN in the acute period mainly manifests as a compact B-line, white lung, or severe AIS, while a double lung point may appear with disease recovery. (ii) Mild or severe TTN is characterized by pleural line abnormalities, A-line disappearance, and different degrees of pleural effusion in one or the bilateral side of chest. (iii) No consolidation is observed in the lung fields (Figure 3).
Pneumonia of the newborn
Pneumonia refers to inflammation of the lung parenchyma, including the terminal airway, alveolar space and pulmonary interstitial areas. It is caused by infectious microorganisms or physical or chemical factors. Pathologically, alveolar inflammatory exudates, hyperemia and edema are present. When bronchiolar epithelium cell necrosis occurs, mucous and cellular debris in the lumen can cause regional air trapping and atelectasis. Pneumonia is responsible for more than 1/3 of all newborn hospitalizations and infectious pneumonia accounts for more than 1/4 of all neonatal deaths especially in the developing world40,41. A meta-analysis showed a sensitivity higher than 96% and specificity higher than 93% when LUS is used to diagnose pneumonia both in adults and children42,43.
LUS imaging characteristics of pneumonia include the following43,44,45,46,47,48. (i) Lung consolidations accompanied by air-bronchograms or fluid-bronchograms; Lung consolidations are the main ultrasound-imaging feature of pneumonia, which are characterized by the following: (a) The size of the consolidation in severe pneumonia is usually large with irregular or jagged boundaries. The shred sign is visible at the edges of the consolidated areas and the dynamic-bronchograms are often visible in severe patients. (b) Consolidations may be located at one or more positions in the lung fields, and consolidated areas may differ in size and shape in the different lung fields. (ii) The pleural line is abnormal and A-lines disappear. (iii) B-lines or AIS are visible in the nonconsolidated areas. (iv) Different degrees of unilateral or bilateral pleural effusion are visible in some infants. (v) The main manifestations of mild or early pneumonia may be presented as small subpleural focal consolidations and AIS (Figure 4).
Meconium aspiration syndrome (MAS) of the newborn
MAS is due to fetal hypoxia leading to defecation and inhalation of meconium-stained amniotic fluid by the infant before or during the delivery process. Meconium particles cause mechanical obstruction of the terminal bronchioles and alveoli together with chemical inflammation and secondary surfactant deficiency. These changes further lead to air-trapping, atelectasis and alveolar or interstitial pulmonary edema. Infants with severe MAS often present with signs of severe respiratory distress including cyanosis, tachypnea, nasal flaring, and retractions and grunting within hours of birth. MAS is a serious lung disease accounting for approximately 10% of all cases of neonatal respiratory failure. Among these patients 10% to 20% will experience pneumothorax and the reported mortality can be as high as 39% in developing and newly industrialized countries49,50.
The bases for the LUS diagnosis of MAS are as follows51,52,53: (i) Lung consolidations accompanied by air-bronchograms are the most important sonogram characteristic of MAS. The scope of consolidation is related to the degree of the disease. The edges of the consolidation area are irregular or jagged and the shred sign is visible. The degrees of consolidation may differ between the two sides of the lung. Similarly, different sizes of consolidation may be present on the same side of the lung. (ii) The pleural line is abnormal, and the A-line disappears. (iii) The B-lines or AIS are visible in the nonconsolidated zone. (iv) Some patients may have different degrees of unilateral or bilateral pleural effusion. It is difficult to differentiate MAS and pneumonia solely based on ultrasound manifestations. Therefore, to obtain a definitive diagnosis it is often necessary to combine ultrasound findings with perinatal history, physical exam and laboratory findings (Figure 5).
Pulmonary hemorrhage of the newborn (PHN)
PHN is not an independent lung disease. In general, it is a late complication of other diseases, its onset is sudden and the infant deteriorates rapidly causing PHN to have a high mortality rate. Pathologically, PHN can present as a focal, regional, or diffuse hemorrhage, usually with alveolar structural damage. The interstitial area of the lung can also be affected. PHN often occurs within the first several days after birth with nearly 90% of PHN occurring within the first week of life54,55.
The main LUS characteristics in PHN are as follows56,57: (i) The shred sign is the most common and the most important LUS sign of PHN. (ii) The degree of lung consolidations accompanied by air-bronchograms are closely related to the severity of the primary diseases. (iii) More than 80% of the patients have different degrees of unilateral or bilateral pleural effusion. Thoracentesis usually confirms the effusion is bleeding. In severe cases, fibrous, cordlike, floating objects formed by fibrin degeneration are visible within the effusion. These objects can be seen floating in the effusion along with respiratory movement by real-time ultrasound. (iv) Miscellaneous signs include pleural line abnormalities, A-line disappearance and AIS (Figure 6).
Pulmonary atelectasis of the newborn
Inadequate aeration resulting from collapse of previously expanded pulmonary tissue is defined as atelectasis49,50. Atelectasis can be divided into obstructive and compressive atelectasis based on the pathophysiology. It can also be divided into complete atelectasis and incomplete atelectasis according to the degree of atelectasis. It is not only an independent disease but rather a common complication of multiple diseases. Atelectasis is a common cause of neonatal respiratory distress and often contributes to prolonged illness or difficulty weaning from ventilator support. Correct diagnosis and appropriate treatment lead to improved outcomes58,59. LUS has a great diagnostic value in cases of pulmonary atelectasis.
Characteristic LUS findings include60,61,62: (i) Lung consolidation accompanied by air bronchograms, or even dynamic bronchograms or parallel air bronchograms are visible in severe cases. (ii) The edges of the consolidation area are relatively clear and regular in severe large-area pulmonary atelectasis. If the atelectasis is limited to a small region, the edges of the consolidation area may not be obvious. (iii) The pleural line in the consolidation area is abnormal and A-lines disappear. (iv) In the early stages of severe or large-area atelectasis, the lung pulse may be visible while lung sliding often disappears under real-time ultrasound. (v) The pulmonary blood flow may be visible in the consolidated areas by color or power Doppler ultrasound. If atelectasis persists (the final stages of atelectasis), both the dynamic bronchograms and the blood flow will disappear (Figure 7, Figure 8, Video 4, Supplemental Video 1, Supplemental Video 2).
Pneumothorax of the newborn
Abnormal accumulation of air in the pleural space is defined as a pneumothorax. It is a relatively common but critical neonatal illness associated with high morbidity and mortality especially in preterm infants63,64. Ultrasound diagnosis of a pneumothorax is very sensitive and specific. Both meta-analysis and prospective controlled studies have shown that LUS is more accurate than CXR for the detection of pneumothorax66,67.
Pneumothorax is diagnosed based on the following LUS signs20,65,66,67,68: (i) Disappearance of lung sliding is the most important sign in the ultrasound diagnosis of pneumothorax. If lung sliding is present, pneumothorax can essentially be excluded. (ii) There are no B-line or comet tail signs, if present pneumothorax can also be excluded. (iii) The clear presence of the lung point is a specific sign for ultrasound diagnosis of mild-to-moderate pneumothorax. However, there is no lung point in severe pneumothorax. The specificity of the lung point in diagnosing pneumothorax is 100% while the sensitivity of approximately 70% or higher21. (iv) The pleural line and A-lines are present. Pneumothorax can be excluded if these lines disappear. (v) On M-mode imaging the sandy beach signs are replaced by the stratosphere signs (Figure 9, Figure 10, Video, 5, Video 6).
For beginners, the following steps may be taken if there are clinical doubts. (i) First, observe the pleural line and the A-line: if they are absent, pneumothorax can be excluded. (ii) If the pleural line and A-lines are present (that is normal lung appearance under B-mode ultrasound), observe lung sliding under real-time ultrasound. If it is present, pneumothorax can be excluded. (iii) If lung sliding disappears, observe the B-line or comet tail sign. If either is present, pneumothorax can be excluded. (iv) If lung sliding disappears and there is no B-line, observe the lung point. If it is present, then mild-to-moderate pneumothorax is essentially confirmed. If it is absent, then severe pneumothorax may have occurred. (v) On M-mode imaging, if the beach sign is replaced by a stratosphere sign, the existence of pneumothorax is further confirmed. The pneumothorax diagnostic procedure is shown in the Figure 11.
Pulmonary edema in cardiac insufficiency
Causes of pulmonary edema in newborns are similar to the ones in the adult population. In addition to the newborns with congenital heart diseases or cardiac insufficiency, many preterm infants with bronchopulmonary dysplasia (BPD) may show signs that are consistent with pulmonary edema69,70. Occasionally, LUS shows an increase in bilateral B-lines or interstitial fluid even before CXR. This pattern may improve upon cardiac treatment or surgery.
Examining correct ETT placement and position
In pediatric and neonatal populations, studies have shown that POC-US is a feasible tool that has been used clinically to verify both correct endotracheal tube (ETT) placement and an acceptable ETT tip position71,72,73,74,75. Proper ETT placement includes both tracheal intubation and an acceptable ETT tip position. Visualization of the ETT tip at a distance ranging from 0.5 to 1.0 cm from the upper border of the aortic arch suggests that the ETT is not too deep. This method has been validated in several studies73. A recent study confirmed these findings and found that ultrasound provided images more rapidly than CXR (mean 19.3 vs. 47 minutes, respectively)72. The concordance of POC-US with CXR to recognize deep and shallow ETT tips was 95%. The sensitivity of LUS to detect deeply positioned ETT tips on X-ray was 86% (specificity of 96%)73. Other studies have evaluated the distance from the ETT tip to the superior aspect of the main pulmonary artery that anatomically corresponds to the level of the carina and found a good correlation between this technique and radiography75,76.
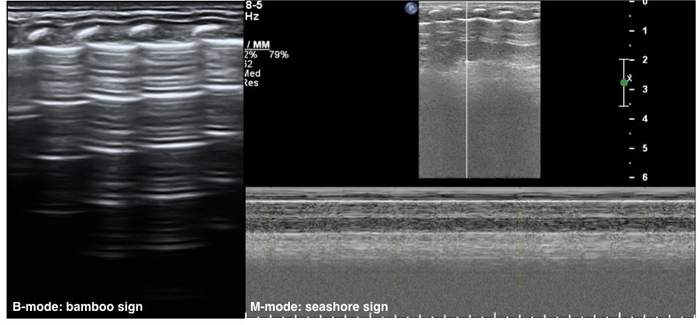
Figure 1: Neonatal normal LUS characteristics.
On B-mode imaging, the pleural line and A-line show smooth, regular and hyperechoic lines arranged in parallel and equidistant from each other, that is bamboo sign. The A-line echoes gradually diminish until they disappear. In M-mode, a seashore sign is present. Please click here to view a larger version of this figure.
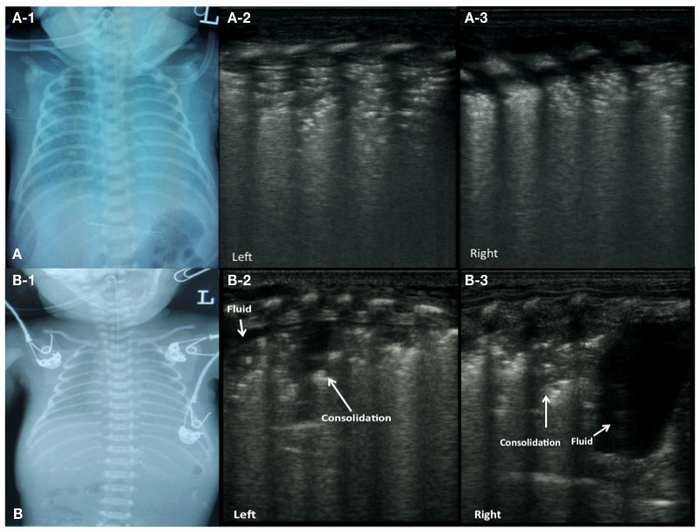
Figure 2: LUS image characteristics of RDS patients.
(A) CXR of a patient with grade II-III RDS (A-1). LUS shows lung consolidation with air bronchograms in bilateral lung fields, disappearance of the pleural line and A-lines (A-2: left lung, A-3: right lung).
(B) CXR of a patient with grade III RDS (B-1). LUS shows a large area of consolidation and a small effusion in the left lung (B-2), significant consolidation in the upper field and a large amount of pleural effusion in the lower field of the right lung (B-3). Please click here to view a larger version of this figure.
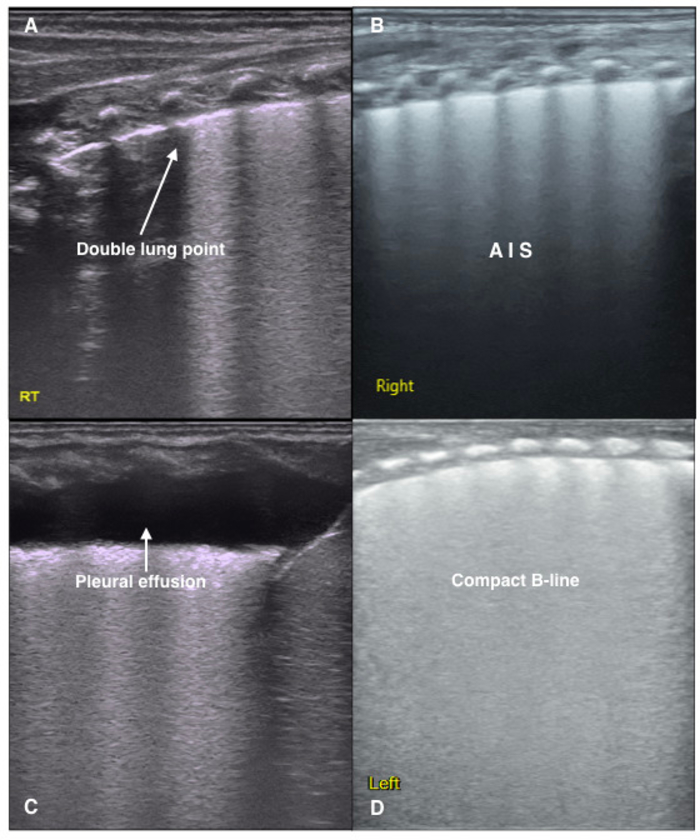
Figure 3: LUS image characteristics of TTN patients.
(A) Double lung point. Clear, sharp cut-off point between the upper and lower lung fields. It is formed when there are differences in the degrees of pathological changes. This sign is often observed in mild TTN.
(B) LUS shows a disappearance of the pleural line and A-lines, as well as AIS in the lung fields.
(C) An area of fluid in the right lung indicating a pleural effusion.
(D) The dense B-line causes the acoustic shadows of the ribs to disappear from the entire scanned area. This type of B-line is called a compact B-line. White lung is defined as the existence of compact B-lines within each lung field. Both compact B-lines and white lung are common ultrasound signs of severe TTN. Please click here to view a larger version of this figure.
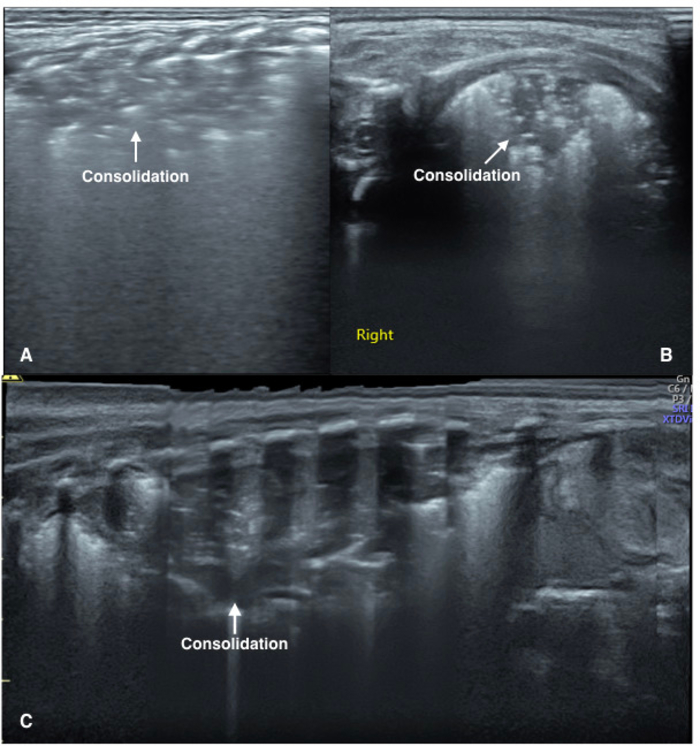
Figure 4: LUS image characteristics of pneumonia patients.
(A) Vertical scanning: The image demonstrates large areas of lung consolidation with air bronchograms in the lung field. The consolidation area has irregular boundaries.
(B) Parallel scanning: The image shows large areas of lung consolidation with significant air bronchograms in the lung field.
(C) Extended view: A severe pneumonia patient. Extended view shows a whole aspect of the consolidations involving the left lung. Please click here to view a larger version of this figure.
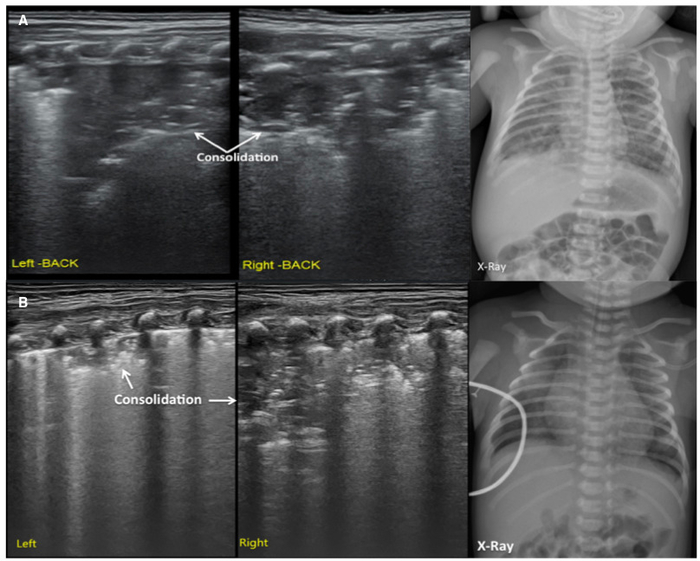
Figure 5: LUS image characteristics of MAS patients.
(A) LUS shows large areas of pulmonary consolidation with irregular edges, especially in the right lung. This finding is consistent with the CXR.
(B) LUS shows a large lung consolidation with the air bronchograms, irregular edges, abnormal pleural line and the absence of A-lines. The CXR shows patchy opacities that highly suggest MAS. Please click here to view a larger version of this figure.
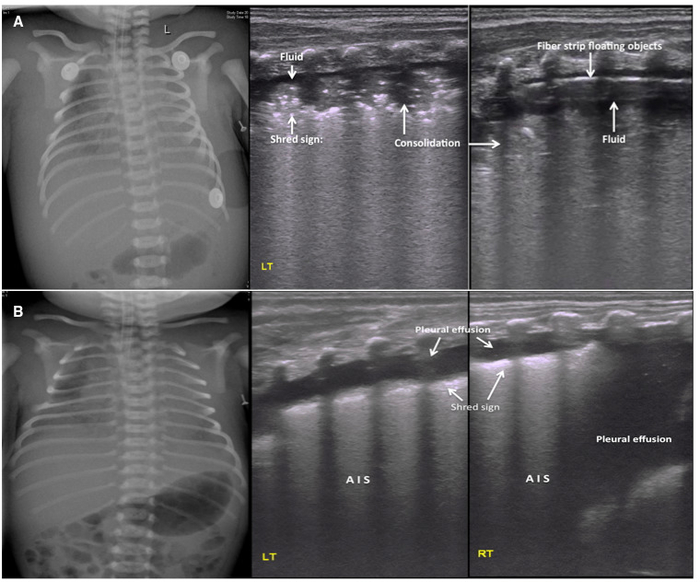
Figure 6: LUS image characteristics of PHN.
(A) Ultrasound findings in a severe PHN patient. CXR shows bilateral hazy lung fields with low lung volumes and pleural effusions. Middle and right: LUS shows a large area of lung consolidation with an air bronchogram, shred sign at the edge of the consolidation and pleural effusions in both sides of the lungs. The pleural effusion confirmed to be hemorrhagic by thoracentesis. Pleural line and A-line are absent. Fibrous protein depositions are observed as cordlike floating objects on real-time ultrasound.
(B) Pleural effusion as the main ultrasound finding in PHN patients. LUS shows significant pleural effusion on both sides of the chest (more severe on the right). This finding is consistent with the CXR. The fluid was confirmed to be bloody by thoracentesis. The other findings are AIS and mild shred signs. Please click here to view a larger version of this figure.
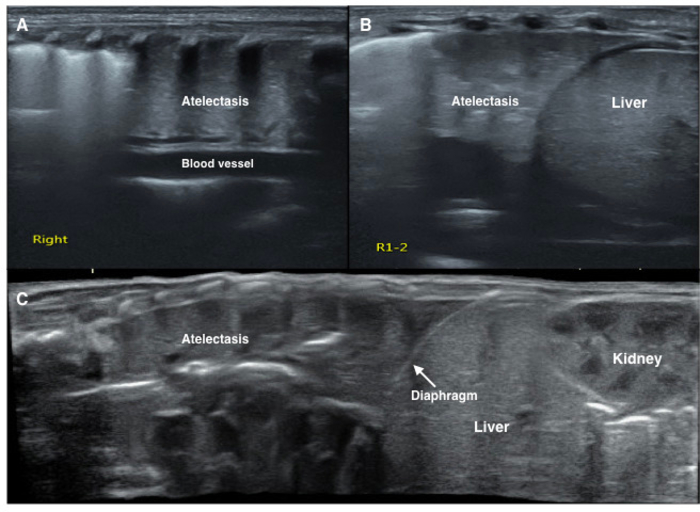
Figure 7: LUS image characteristics of pulmonary atelectasis of the newborn.
LUS shows a large consolidation area with regular edges in the right lung (A, B, C). The echogenicity of the consolidated lung tissue is similar to that of the adjacent liver tissue (B, C). Significant air bronchograms are observed (C). Please click here to view a larger version of this figure.
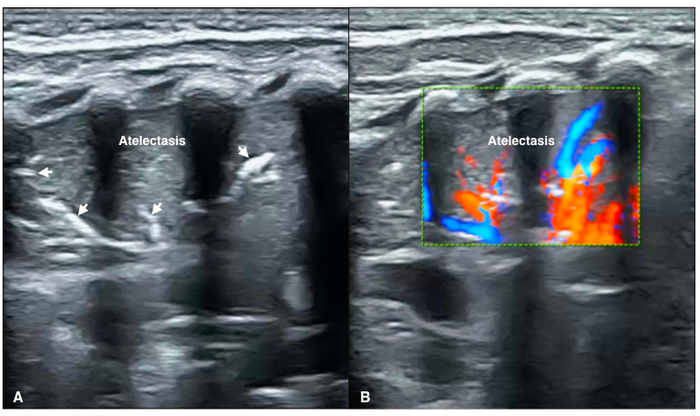
Figure 8: Blood flow within atelectasis
(A) B-mode LUS shows a large area consolidation with a significant air-bronchograms (arrow) as well as regular margins, presented as atelectasis.
(B) Color Doppler ultrasound shows significant arterial blood supply within consolidated area of the lung (Video 4). Please click here to view a larger version of this figure.
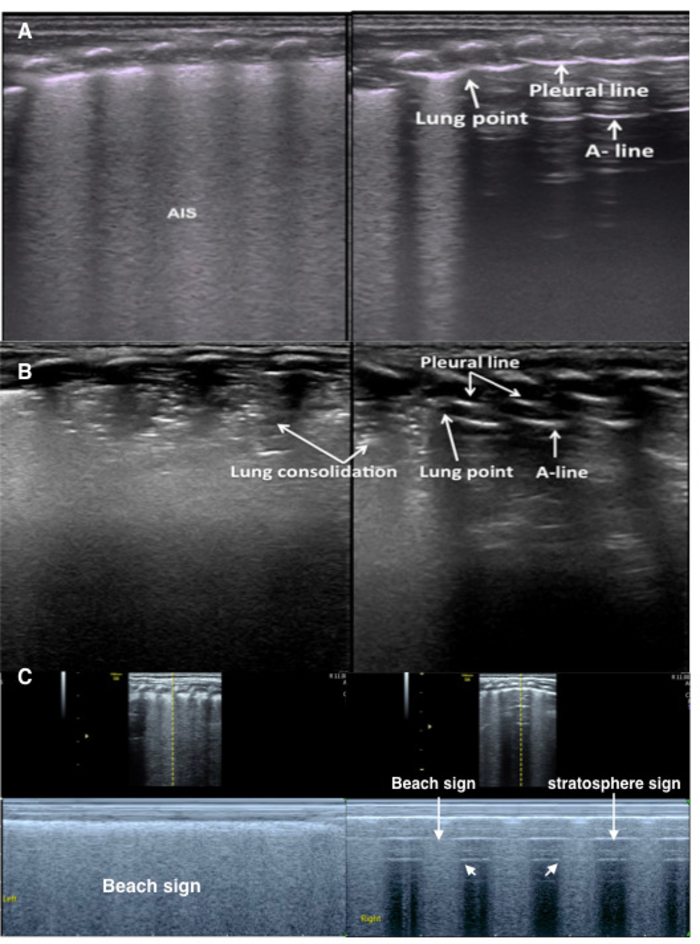
Figure 9: Lung point in mild-moderate pneumothorax
(A) TTN patient with pneumothorax. The B-mode LUS shows an abnormal pleural line, AIS and disappearing A-lines in the left lung. Right lung shows a lung point. Lung sliding occurs in the B-line area but is absent in the A-line area on real-time ultrasound (Video 5).
(B) RDS patient with pneumothorax. B-mode LUS shows a large lung consolidation with air bronchograms in the left lung and a small consolidation in the right lung. The pleural line and A-lines are present on the right side of the right lung.
(C) Lung point under M-mode ultrasound. Left lung shows the beach sign. Right lung shows the lung point (the point between beach sign and stratosphere sign), confirming mild pneumothorax. Please click here to view a larger version of this figure.
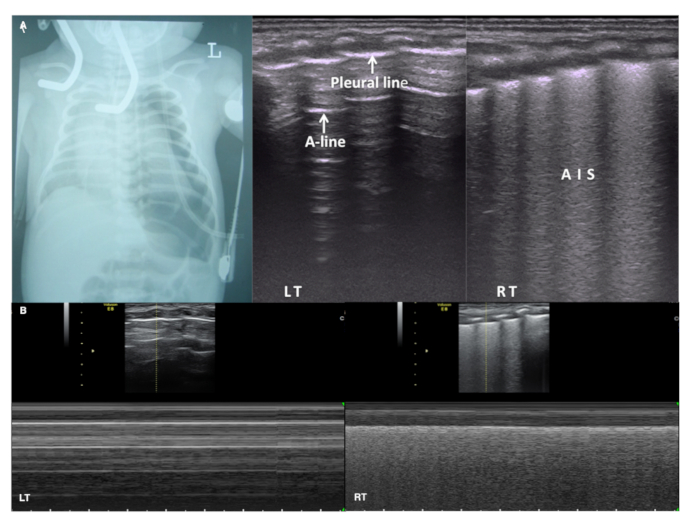
Figure 10: LUS in massive pneumothorax
(A) CXR shows severe pneumothorax in the left lung. Pleural line and A-lines are present on the left lung but no lung point is found. LUS shows AIS in the right lung. Lung sliding disappears in the whole left lung field while present on the right on real-time ultrasound (Video 6).
(B) Under M-mode ultrasound the right lung shows a beach sign while the left lung presents a stratosphere sign (also known as a barcode sign). This confirms a severe pneumothorax in the left hemithorax. Please click here to view a larger version of this figure.
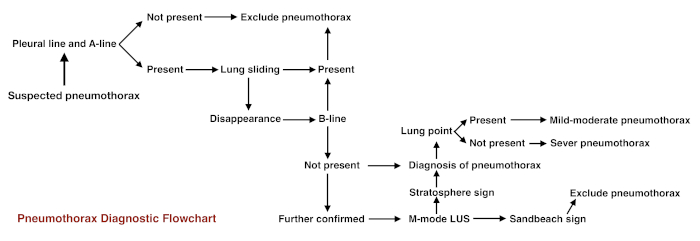
Figure 11: Flowchart for pneumothorax diagnostic procedure Please click here to view a larger version of this figure.
Video 1: Lung sliding
The pleural line moves in synchrony with respirations. Please click here to view this video. (Right-click to download.)
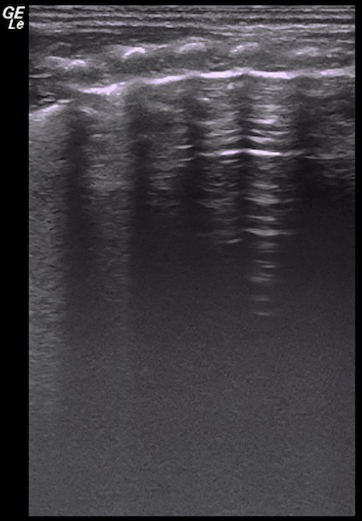
Video 2: Dynamic air bronchograms
When severe lung consolidation is present air-bronchograms move with the respirations. This kind of air-bronchogram is also known as dynamic air-bronchogram. Please click here to view this video. (Right-click to download.)
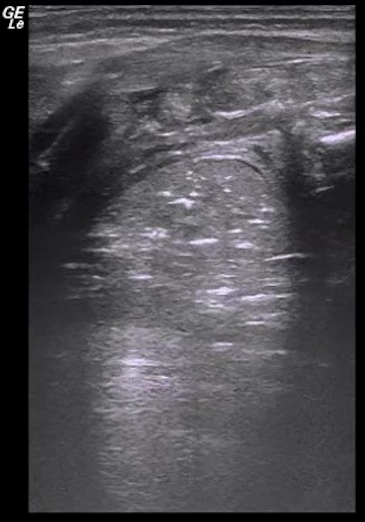
Video 3: Lung pulse
If the area of lung consolidation is large enough, the consolidated lung pulsates in synchrony with heartbeats, this kind of pulsation is called the lung pulse. Please click here to view this video. (Right-click to download.)
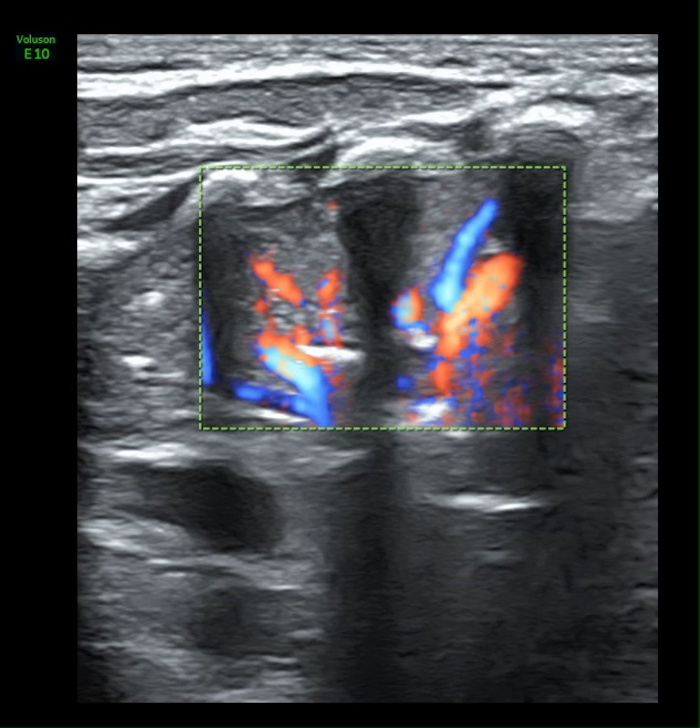
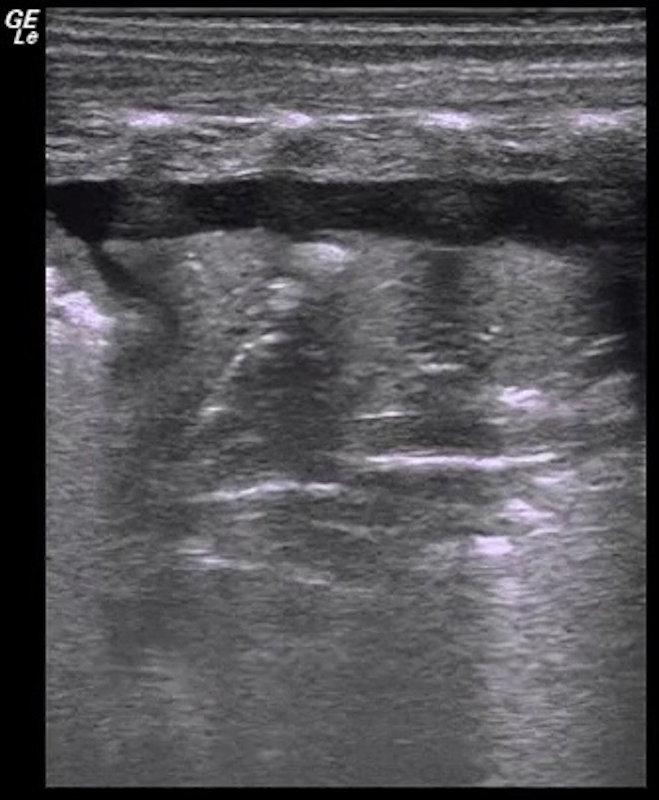
Video 4: Blood supply in atelectasis area
The rich blood supplying can be found under Color Doppler ultrasound. Please click here to view this video. (Right-click to download.)
Video 5: Lung point in a mild-moderate pneumothorax patient
Lung sliding occurs in the B-line area but is absent in the A-line area on real-time ultrasound. Please click here to view this video. (Right-click to download.)
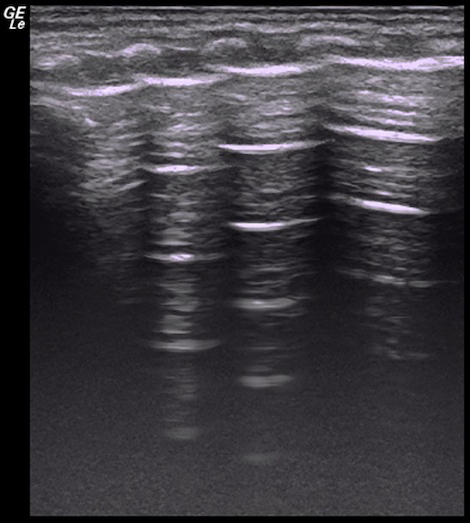
Videos 6: Disappeared lung sliding in a severe pneumothorax patient
Lung sliding disappeared in the entire right lung field. It is presented in the left lung. Please click here to view this video. (Right-click to download.)
Supplemental Figure 1: Pleural line
Under B-mode ultrasound, the pleural line appears as a smooth, regular hyperechoic lines. Please click here to download this file.
Supplemental Figure 2: A-lines
A-lines are situated below the pleural line. They present as a series of smooth, linear hyperechoic parallel lines. Please click here to download this file.
Supplemental Figure 3: B-line, confluent B-line, and AIS
(A) B-lines. B-lines arise from and are roughly vertical to the pleural line.
(B) Confluent B-lines. Confluent B-lines occur when the entire intercostal space is full of intense B-lines, but the acoustic shadow of the ribs is still clearly displayed.
(C) Alveolar-interstitial syndrome. AIS is defined by the presence of two or more sequential intercostal spaces with confluent B-lines in any scanning area. Please click here to download this file.
Supplemental Figure 4: Compact B-lines.
Compact B-lines refer to the concentration of B-lines that causes acoustic shadow of the ribs to disappear within the scanning zone. White lung occurs when each scanning zone on both sides of the lung presents as compact B-lines. Please click here to download this file.
Supplemental Figure 5: Lung consolidation and shred sign.
(A) Lung consolidation. On LUS lung tissues gives appearance of tissue-like density, also called ‘hepatization’ of the lung.
(B) Shred sign. When the boundary between consolidated lung tissue and aerated lung tissue is unclear the ultrasound sign formed between the two areas is called a shred sign. Please click here to download this file.
Supplemental Figure 6: Lung point
The transition point from the B-line area to the parietal pleura and A-line existing area is the lung point. Please click here to download this file.
Supplemental Figure 7: Double lung point.
Differences in the degree or pathologic changes between upper and lower lung fields indicate a double lung point. Please click here to download this file.
Supplemental Figure 8 Sandy beach sign and stratosphere sign
Under M-mode ultrasound, the part A presents the sandy beach sign (generally excluded pneumothorax) while the part B shows the stratosphere sign (generally seen in pneumothorax). Please click here to download this file.
Supplemental Video 1: Lung pulse in a patient with severe atelectasis
Severe atelectasis in the left lung. Movement of the atelectatic lung can be observed with the heart beat by real-time ultrasound; this movement is called the lung pulse. Please click here to download this file.
Supplemental Video 2: Dynamic air bronchograms in a patient with severe atelectasis
Air bronchograms are observed with respiratory movement by real-time ultrasound. This kind of movement is known as a dynamic air bronchogram and is a common ultrasound sign in severe atelectasis patients. Please click here to download this file.
Discussion
POC-LUS is a feasible and convenient diagnostic method that can be performed in the NICU at the bedside. It is very sensitive and reliable in the diagnosis of all types of neonatal lung diseases77. Furthermore, it has many advantages over the CXR and CT scan such as accuracy, reliability, low cost, simplicity and no risk of adverse effects due to radiation. Therefore, we encourage the use of LUS in the NICU. When learning this imaging modality, the following issues need to be carefully considered: (1) Examiners require at least 6-8 weeks of training. They have to evaluate 20-30 patients with each type of lung disease to master the technique. The diagnostic sequence for pneumothorax is more challenging in neonates compared to older children or adults. We suggest that in this case trainees receive extra training time. (2) Examiners operate in strict accordance with the operating procedures of the ultrasound instrument. (3) Examiners should reduce adverse stimulation of neonate as much as possible. The ultrasound exam is to be performed at appropriate times, especially in high-risk infants. (4) The exam is to be ideally performed with a quiet and calm neonate. No sedatives are needed to perform the examination. (5) Care must be taken to keep the neonate warm. Ultrasound gel must be preheated. (6) Sterilization and isolation procedures must be observed. The operators should wash their hands, carefully clean and sterilize the probe and use a protective plastic probe cover to avoid cross-contamination.
Perpendicular scanning is the most important and most commonly used scanning method. Since sub-pleural lung tissue is located at the distal end of the bronchial and blood supply, it is more likely to be affected by different lung diseases. Therefore, perpendicular scanning can delineate nearly the entire lung anatomy in neonates. Certainly, parallel scanning is also very helpful in detecting mild lung lesions (i.e., pathological changes involving only 1-2 intercostal spaces and limited to the subpleural areas) or in identifying the "lung point" when a mild-moderate pneumothorax is suspected10. When the lesions mainly involve the bottom of the bilateral lungs, scanning may also be performed below the diaphragm via the liver as an acoustic window. This type of scanning can also be used to examine the integrity of the diaphragm and the presence of pleural effusions.
In clinical practice, however, LUS examination should not be limited to a fixed scanning sequence. The scan can be performed from the most convenient place based on the infant's position during the examination. Starting LUS scanning from the back is acceptable and easy to perform. It also avoids interference from the heart and the large vessels. Further scanning in other areas of the lungs must be performed in any infant with high suspicion of a pulmonary lesion in a situation where scanning of the back reveals no abnormalities.
Occasionally, we may use the extended view (XTD-View) function. The XTD-View function can construct an extended image from individual image frames as the operator slides the transducer along the narrow axis of the probe. XTD-View allows the doctors to assess the interesting areas and neighboring structures fully (Figure 4C). To do this, we should orient the transducer parallel to the direction of transducer motion before activating the XTD-View button. It is necessary to slide the transducer towards the notch and keep the transducer perpendicular to the ribs during the whole scanning.
LUS has some limitations. (1) It is highly operator dependent. Therefore, it is necessary to gain sufficient experience to fully understand the basic principles of LUS before performing examinations. (2) Subcutaneous emphysema affects the image quality as well as accuracy of the results, thus it may interfere with scanning. (3) The role of LUS in emphysema, pneumomediastinum and the diagnosis of bronchopulmonary dysplasia remains uncertain. (4) Some mild cases may be missed if the scanning is not performed carefully. (5) It was reported that LUS has a limited value as a diagnostic tool for rare cystic lung diseases, such as lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis and Birt-Hogg-Dubé syndrome78.
Current literatures offer well-designed, systematic and in depth research in the area of LUS. Research findings have been validated and confirmed in clinical practice. Our protocol and guidelines have been developed after a thorough evidence-based review of the currently available data by a panel of international experts in this field.
開示
The authors have nothing to disclose.
Acknowledgements
We acknowledge all the experts and authors that participated in writing the manuscript. This work was supported by the Foundation of Beijing Chaoyang District Committee of Science and Technology (CYSF1820) and the Clinical Research Special Fund of Wu Jieping Medical Foundation (320. 6750. 15072).
We acknowledge the Division of Perinatology, the Society of Pediatrics of the Chinese Medical Association and the Division of Neonatal Ultrasound Society, the Chinese Neonatologist Association as well as the Chinese College of Critical Ultrasound for organizing this work.
We acknowledge the all the staff who worked for the Department of Neonatology and the NICU, Beijing Chaoyang District Maternal and Child Healthcare Hospital, especially the nursing group who gave great assistant to this work, particularly during the process of the video recording.
Materials
| Ultrasound machine | GE Healthcare | H44792LW | Ultrasound machine,Voluson S10 BT16,Probe ML6-15 & 9L |
| Ultrasound machine | GE Healthcare | H48701UZ | Ultrasound machine,Voluson E10 BT18 OLED,Probe ML6-15 & 9L |
| Ultrasound machine | Philips Healthcare | US818C0258 | Ultrasound machine,EpiQ5,Probe L18-5 |
| Ultrasound machine | Philips Healthcare | US715F1270 | Ultrasound machine,Affiniti70,Probe eL4-18 |
| Ultrasound gel | Tianjin Xiyuansi Company | TM20160195 | Aquasonic 100 ultrasound transmission gel |
| Disinfection wipe | Nantong Sirui Company Ltd. | YZB0016-2013 | Benzalkonium Bromide Patches |
参考文献
- Chavez, M. A., et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respiratory Research. 15, 50 (2014).
- Yilmaz, H. L., Özkaya, A. K., Gökay, S. S., Kendir, &. #. 2. 1. 4. ;. T., Şenol, H. Point-of-care lung ultrasound in children with community acquired pneumonia. The American Journal of Emergency Medicine. 35 (7), 964-969 (2017).
- Volpicelli, G., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Medicine. 38 (4), 577-591 (2012).
- Hiles, M., Culpan, A. M., Watts, C., Munyombwe, T., Wolstenhulme, S. Neonatal respiratory distress syndrome: chest X-ray or lung ultrasound? A systematic review. Ultrasound. 25 (2), 80-91 (2017).
- Liu, J., Cao, H. Y., Wang, X. L., Xiao, L. J. The significance and the necessity of routinely performing lung ultrasound in the neonatal intensive care units. The Journal of Maternal-Fetal & Neonatal Medicine. 29 (24), 4025-4030 (2016).
- Cattarossi, L., Copetti, R., Poskurica, B. Radiation exposure early in life can be reduced by lung ultrasound. Chest. 139 (3), 730-731 (2011).
- Kurepa, D., Zaghloul, N., Watkins, L., Liu, J. Neonatal lung ultrasound exam guidelines. Journal of Perinatology. 38 (1), 11-22 (2018).
- Chen, S. W., Fu, W., Liu, J., Wang, Y. Routine application of lung ultrasonography in the neonatal intensive care unit. 医学. 96 (2), e5826 (2017).
- Lichtenstein, D. A., Mauriat, P. Lung ultrasound in the critically ill neonate. Current Pediatric Reviews. 8 (3), 217-223 (2012).
- Liu, J., Ren, X. L., Fu, W., Liu, Y., Xia, R. M. Bronchoalveolar lavage for the treatment of neonatal pulmonary atelectasis under lung ultrasound monitoring. The Journal of Maternal-Fetal & Neonatal Medicine. 30 (19), 2362-2366 (2016).
- Liu, J., Ren, X. L., Li, J. J. POC-LUS Guiding Pleural Puncture Drainage to Treat Neonatal Pulmonary Atelectasis Caused by Congenital Massive Effusion. The Journal of Maternal-Fetal & Neonatal Medicine. , (2018).
- Lichtenstein, D. A., Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Chest. 108 (5), 1345-1348 (1995).
- Piette, E., Daoust, R., Denault, A. Basic concepts in the use of thoracic and lung ultrasound. Current Opinion in Anaesthesiology. 26 (1), 20-30 (2013).
- Volpicelli, G., et al. Detection of sonographic B-lines in patients with normal lung or radiographic alveolar consolidation. Medical Science Monitor. 14 (3), 122-128 (2008).
- Dietrich, C. F., et al. Lung B-line artefacts and their use. Journal of Thoracic Disease. 8 (6), 1356-1365 (2016).
- Copetti, R., Cattarossi, L., Macagno, F., Violino, M., Furlan, R. Lung ultrasound in respiratory distress syndrome: a useful tool for early diagnosis. Neonatology. 94 (1), 52-59 (2008).
- Lichtenstein, D. A., Lascols, N., Mezière, G., Gepner, A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Medicine. 30 (2), 276-281 (2004).
- Touw, H. R., Tuinman, P. R., Gelissen, H. P., Lust, E., Elbers, P. W. Lung ultrasound: routine practice for the next generation of internists. Netherlands Journal of Medicine. 73 (3), 100-107 (2015).
- Lichtenstein, D. A., Lascols, N., Prin, S., Mezière, G. The "lung pulse": an early ultrasound sign of complete atelectasis. Intensive Care Medicine. 29 (12), 2187-2192 (2003).
- Lichtenstein, D., Mezière, G., Biderman, P., Gepner, A. The "lung point": an ultrasound sign specific to pneumothorax. Intensive Care Medicine. 26 (10), 1434-1440 (2000).
- Copetti, R., Cattarossi, L. The double lung point: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology. 91 (3), 203-209 (2007).
- Lichtenstein, D. A., et al. Ultrasound diagnosis of occult pneumothorax. Critical Care Medicine. 33 (6), 1231-1238 (2005).
- Liu, J. Lung ultrasonography for the diagnosis of neonatal lung disease. Journal of Maternal-Fetal Neonatal Medicine. 27 (8), 856-861 (2014).
- Chen, S. W., Zhang, M. Y., Liu, J. Application of Lung Ultrasonography in the Diagnosis of Childhood Lung Diseases. Chinese Medical Journal. 128 (19), 2672-2678 (2015).
- Koivisto, M., et al. Changing incidence and outcome of infants with respiratory distress syndrome in the 1990s: a population-based survey. Acta Paediatrica. 93 (2), 177-184 (2004).
- Ayachi, A., et al. Hyaline membrane disease in full-term neonates. Archives de Pediatrie. 12 (20), 156-159 (2005).
- Liu, J., et al. Clinical characteristics, diagnosis and management of respiratory distress syndrome in full-term neonates. Chinese Medical Journal. 123 (19), 2640-2644 (2010).
- Zhang, Y., Li, P. Meta-analysis of lung ultrasound for the diagnosis of neonatal respiratory distress syndrome. China Medical Herald. 14 (24), 139-142 (2017).
- Lovrenski, J. Lung ultrasonography of pulmonary complications in preterm infants with respiratory distress syndrome. Upsala Journal of Medical Sciences. 117 (1), 10-17 (2012).
- Lovrenski, J., Sorantin, E., Stojanovic, S., Doronjski, A., Lovrenski, A. Evaluation of surfactant replacement therapy effects: a new potential role of lung ultrasound. Srpski Arhiv za Celokupno Lekarstvo. (11-12), 669-675 (2015).
- Liu, J., Cao, H. Y., Wang, H. W., Kong, X. Y. The role of lung ultrasound in diagnosis of respiratory distress syndrome in newborn infants. Iranian Journal of Pediatrics. 24 (2), 147-154 (2014).
- Liu, J., Cao, H. Y., Liu, Y. Lung ultrasonography for the diagnosis of neonatal respiratory distress syndrome: a pilot study. Chinese Journal of Pediatrics. 51 (3), 205-210 (2013).
- Liu, J., Wang, Y., Fu, W., Yang, C. S., Huang, J. J. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. 医学. 93 (27), e197 (2014).
- Vergine, M., Copetti, R., Brusa, G., Cattarossi, L. Lung ultrasound accuracy in respiratory distress syndrome and transient tachypnea of the newborn. Neonatology. 106 (2), 87-93 (2014).
- Abu-Shaweesh, J. M., Martin, R. J., Fanaroff, A. A., Walsh, M. C. Respiratory disorder in preteen and term infants. Fanaroff and Martin’s Neonatal-Perinatal. , 1141-1170 (2011).
- Greenough, A., Greenough, A., Milner, A. D. Transient tachypnea of the newborn. Neonatal Respiratory Disorder. , 272-277 (2003).
- Rocha, G., Rodrigues, M., Guimarães, H. Respiratory distress syndrome of the preterm neonate-placenta and necropsy as witnesses. Journal of Maternal-Fetal & Neonatal Medicine. 24 (1), 148-151 (2011).
- Liu, J., et al. Lung ultrasonography to diagnose transient tachypnea of the newborn. Chest. 149 (5), 1269-1275 (2016).
- Liu, J., et al. Value of lung ultrasound on diagnosing transient tachypnea of the newborn. Journal of Applied Clinical Pediatrics. 31 (2), 93-96 (2016).
- Duke, T. Neonatal pneumonia in developing countries. Archives of Disease in Childhood – Fetal and Neonatal Edition. 90 (3), 211-219 (2005).
- Rao, Y. Analysis of death reasons in 1509 newborn infants. Zhongguo Fuyou Jiankang Yanjiu. 20 (5), 686-688 (2009).
- Chavez, M. A., et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respiratory Research. 15, (2014).
- Pereda, M. A., et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 135 (4), 714-722 (2015).
- Caiulo, V. A., et al. Lungultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatric Pulmonology. 48 (3), 280-287 (2013).
- Reissig, A., et al. Lung Ultrasound in the Diagnosis and Follow-up of Community-Acquired Pneumonia: A Prospective, Multicenter, Diagnostic Accuracy Study. Chest. 142 (4), 965-972 (2012).
- Liu, J., Liu, F., Liu, Y., Wang, H. W., Feng, Z. C. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest. 146 (2), 383-388 (2014).
- Liu, J., et al. Value of lung ultrasound in diagnosing infectious pneumonia of newborns. Chinese Journal of Perinatal Medicine. 17 (7), 468-472 (2014).
- Rodríguez-Fanjul, J., Balcells, C., Aldecoa-Bilbao, V., Moreno, J., Iriondo, M. Lung ultrasound as a predictor of mechanical ventilation in neonates older than 32 weeks. Neonatology. 110 (3), 198-203 (2016).
- Swarnam, K., Soraisham, A. S., Sivanandan, S. Advances in the management of meconium aspiration syndrome. International Journal of Pediatrics. 2012, 359571 (2012).
- Van Ierland, Y., De Beaufort, A. J. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Human Development. 85 (10), 617-620 (2009).
- Piastra, M., et al. Lung ultrasound findings in meconium aspiration syndrome. Early Human Development. 90, S41-S43 (2014).
- Liu, J., Cao, H. Y., Fu, W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. Journal of International Medical Research. 44 (6), 1534-1542 (2016).
- Liu, J., et al. Lung ultrasonography for the diagnosis of meconium aspiration syndrome of the newborn infants. Chinese Journal of Applied Clinical Pediatrics. 31 (16), 1227-1230 (2016).
- Zahr, R. A., Ashfaq, A., Marron-Corwin, M. Neonatal pulmonary hemorrhage. NeoReviews. 13 (5), e302-e306 (2012).
- Berger, T. M., Allred, E. N., Van Marter, L. J. Antecedents of clinically significant pulmonary hemorrhage among newborn infants. Journal of Perinatology. 20 (5), 295-300 (2000).
- Liu, J., et al. The diagnosis of neonatal pulmonary hemorrhage using lung ultrasound. Chinese Journal of Pediatrics. 55 (1), 46-49 (2017).
- Ren, X. L., Fu, W., Liu, J., Liu, Y., Xia, R. M. Lung ultrasonography to diagnose pulmonary hemorrhage of the newborn. The Journal of Maternal-Fetal & Neonatal Medicine. 30 (21), 2601-2606 (2017).
- Johnston, C., Carvalho, W. B. Atelectasis: mechanisms, diagnosis and treatment in the pediatric patient. Revista da Associação Médica Brasileira. 54 (5), 455-460 (2008).
- Nakos, G., Tsangaris, H., Liokatis, S., Kitsiouli, E., Lekka, M. E. Ventilator-associated pneumonia and atelectasis: evaluation through bronchoalveolar lavage fluid analysis. Intensive Care Medicine. 29 (4), 555-563 (2003).
- Liu, J., et al. Lung ultrasound for diagnosis of neonatal atelectasis. Chinese Journal of Pediatrics. 51 (9), 644-648 (2013).
- Liu, J., et al. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest. 147 (4), 1013-1019 (2015).
- Lichtenstein, D., Mezière, G., Seitz, J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 135 (6), 1421-1425 (2009).
- Duong, H. H., et al. Pneumothorax in neonates: trends, predictors and outcomes. Journal of Neonatal-Perinatal Medicine. 7 (1), 29-38 (2014).
- Bhatia, R., Davis, P. G., Doyle, L. W., Wong, C., Morley, C. J. Identification of pneumothorax in very preterm infants. The Journal of Pediatrics. 159 (1), 115-120 (2011).
- Alrajab, S., et al. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Critical Care. 17, R208 (2013).
- Cattarossi, L., Copetti, R., Brusa, G., Pintaldi, S. Lung ultrasound diagnostic accuracy in neonatal pneumothorax. Canadian Respiratory Journal. 2016, 6515069 (2016).
- Raimondi, F., et al. Lung ultrasound for diagnosing pneumothorax in the critically ill neonate. The Journal of Pediatrics. 175, 74-78 (2016).
- Liu, J., et al. Lung ultrasonography to diagnose pneumothorax of the newborn. The American Journal of Emergency Medicine. 35 (9), 1298-1302 (2017).
- Rodríguez-Fanjul, J., Moreno Hernando, L., Sánchez-de-Toledo, J. Lung ultrasound for cardiogenic shock in VA-ECMO. Revista Española de Cardiología (English Edition). 71 (5), 393 (2017).
- Rodríguez-Fanjul, J., et al. Usefulness of lung ultrasound in neonatal congenital heart disease (LUSNEHDI): lung ultrasound to assess pulmonary overflow in neonatal congenital heart disease. Pediatric Cardiology. 37 (8), 1482-1487 (2016).
- Quintela, P. A., et al. Usefulness of bedside ultrasound compared to capnography and X-ray for tracheal intubation. Anales de Pediatría. 81 (5), 283-288 (2014).
- Oulego-Erroz, I., Alonso-Quintela, P., Rodríguez-Blanco, S., Mata-Zubillaga, D., Fernández-Miaja, M. Verification of endotracheal tube placement using ultrasound during emergent intubation of a preterm infant. Resuscitation. 83 (6), e143-e144 (2012).
- Chowdhry, R., Dangman, B., Pinheiro, J. M. B. The concordance of ultrasound technique versus X-ray to confirm endotracheal tube position in neonates. Journal of Perinatology. 35 (7), 481-484 (2015).
- Sethi, A., Nimbalkar, A., Patel, D., Kungwani, A., Nimbalkar, S. Point of care ultrasonography for position of tip of endotracheal tube in neonates. Indian Pediatrics. 51 (2), 119-121 (2014).
- Sharma, D., Tabatabaii, S. A., Farahbakhsh, N. Role of ultrasound in confirmation of endotracheal tube in neonates: a review. The Journal of Maternal-Fetal & Neonatal Medicine. , (2017).
- Dennington, D., Vali, P., Finer, N. N., Kim, J. H. Ultrasound confirmation of endotracheal tube position in neonates. Neonatology. 102 (3), 185-189 (2012).
- Sharma, D., Farahbakhsh, N. Role of chest ultrasound in neonatal lung diseases: a review of current evidences. The Journal of Maternal-Fetal & Neonatal Medicine. 32 (2), 310-316 (2019).
- Davidsen, J. R., Bendstrupd, E., Henriksen, D. P., Graumanne, O., Laursena, C. B. Lung ultrasound has limited diagnostic value in rare cystic lung diseases:a cross-sectional study. European Clinical Respiratory Journal. 4 (1), 1330111 (2017).

