Measurement of Mitochondrial Oxygen Consumption in Permeabilized Fibers of Drosophila Using Minimal Amounts of Tissue
概要
In this paper, a method to measure oxygen consumption using high-resolution respirometry in permeabilized thoraxes of Drosophila is described. This technique requires a minimal amount of tissue compared to the classic mitochondrial isolation technique and the results obtained are more physiologically relevant.
Abstract
The fruit fly, Drosophila melanogaster, represents an emerging model for the study of metabolism. Indeed, drosophila have structures homologous to human organs, possess highly conserved metabolic pathways and have a relatively short lifespan that allows the study of different fundamental mechanisms in a short period of time. It is, however, surprising that one of the mechanisms essential for cellular metabolism, the mitochondrial respiration, has not been thoroughly investigated in this model. It is likely because the measure of the mitochondrial respiration in Drosophila usually requires a very large number of individuals and the results obtained are not highly reproducible. Here, a method allowing the precise measurement of mitochondrial oxygen consumption using minimal amounts of tissue from Drosophila is described. In this method, the thoraxes are dissected and permeabilized both mechanically with sharp forceps and chemically with saponin, allowing different compounds to cross the cell membrane and modulate the mitochondrial respiration. After permeabilization, a protocol is performed to evaluate the capacity of the different complexes of the electron transport system (ETS) to oxidize different substrates, as well as their response to an uncoupler and to several inhibitors. This method presents many advantages compared to methods using mitochondrial isolations, as it is more physiologically relevant because the mitochondria are still interacting with the other cellular components and the mitochondrial morphology is conserved. Moreover, sample preparations are faster, and the results obtained are highly reproducible. By combining the advantages of Drosophila as a model for the study of metabolism with the evaluation of mitochondrial respiration, important new insights can be unveiled, especially when the flies are experiencing different environmental or pathophysiological conditions.
Introduction
The fruit fly, Drosophila melanogaster, has been used as a model organism for genetic research for over a century1. The study of this organism has not only led to significant fundamental knowledge about sex-linked inheritance2, mutation rate3, the development of neural system and the cell fate determination4, but has also recently emerged as a valuable tool to study the mechanisms inherent to several diseases such as Alzheimer's and Parkinson's5,6. Moreover, it is a popular model to study the aging process, as they can be raised in large number over a short period of time and have a short lifespan. They also possess homologous structures to human organs, such as a heart, oenocytes (hepatocyte-like cells), fat bodies (functioning similarly as the liver and white adipose tissue), insulin producing cells (equivalent to β-pancreatic cells), as well as the hemolymph transporting metabolites (analogous to the blood of vertebrates)7. Moreover, the central pathways of intermediary metabolism (including the insulin/insulin-like growth factor-like signaling pathway and Target of Rapamycin-TOR pathways) are also highly conserved7. For these reasons, Drosophila have recently been exploited to describe the fundamental mechanisms that control metabolism, especially in pathological conditions inherent to human metabolic diseases such as diabetes8. A major component of the metabolism is the mitochondrion that integrates multiple pathways and performs one of life's most important biological functions, ATP production, via the oxidative phosphorylation process (OXPHOS). Considering their central role in organism's metabolism, it is not surprising that mitochondrial dysfunctions are involved in many diseases like Parkinson's9 and Alzheimer's diseases10, as well as in amyotrophic lateral sclerosis11,12. They are also fundamental determinants of the aging process. Indeed, they are the main producers of reactive oxygen species (ROS) in the cell, which can be detrimental to the cell at high concentration through oxidative damages11. Aging has also been associated to the accumulation of damaged or mutated mitochondrial DNA13, mitophagy dysfunctions14,15 as well as impairment of mitochondrial biogenesis16. Mitochondria are also key determinants of the cell's homeostasis as they can utilize different substrates to adjust several cellular functions according to the abundance or scarcity of macronutrients17,18.
Indeed, the different nutrients in the diet (carbohydrates, lipids and proteins) are digested, absorbed and transported in the cells. They are then transformed in the cytosol, and the derived substrates are transported into the mitochondrial matrix where they produce reducing equivalents, such as NADH and FADH219. These reducing equivalents are then oxidized by different enzymatic complexes of the electron transport system (ETS). These complexes are embedded in the mitochondrial inner membrane, such as Complex I and Complex II. Additionally, other enzymatic complexes such as the mitochondrial glycerol-3-phosphate dehydrogenase and the proline dehydrogenase represent alternate routes for the entry of electrons into the ETS20,21. These 'alternative' complexes are particularly important in insects, as according to the species, they can actively participate to increase the respiration20,22,23,21. Electrons from these ETS feeding systems are transferred to the ubiquinone and subsequently to Complex III, and then to Complex IV, until the final acceptor, molecular oxygen. This electron transfer generates a proton-motive force across the inner mitochondrial membrane driving the phosphorylation of ADP to ATP at Complex V (Figure 1). Considering the central role of mitochondria in cell's homeostasis, studying mitochondrial metabolism using the relevant model D. melanogaster represents a powerful tool to delineate the underlying mechanisms of various pathophysiological conditions or under cellular and environmental stresses. Surprisingly however, only a handful of studies actually measured mitochondrial respiration in Drosophila24,25,26. Indeed, experiments aiming to evaluate mitochondrial oxygen consumption require the isolation of mitochondria. Although advantageous for the measurement of different mitochondrial functions (such as ROS production or P/O ratio as marker of mitochondrial efficiency27,28), these isolations generally require rather large quantities of tissue from several individuals24,29. This requirement for high amounts of tissue and individuals is an important limiting factor, especially considering that all individuals should be the same age and preferably of the same sex for the experiments, making the measure of respiration at different time points laborious at best. Moreover, while mitochondrial isolations can provide significant insight into the fundamental mechanisms governing mitochondrial metabolism, the methods used to isolate mitochondria have several drawbacks such as the difficulty to obtain replicable results, disruption of the mitochondrial network, and alteration of mitochondrial structure and function29,30,31.
The aim of this study is to present a robust protocol to measure mitochondrial oxygen consumption in Drosophila using only a minimal amount of tissue from very few individuals. This protocol consists of measuring mitochondrial oxygen consumption in situ using permeabilized muscle fibers29 from Drosophila thoraxes in combination with high-resolution respirometry32,33,34,35. This method has also additional advantages compared to the classic mitochondrial isolation method since the interactions with the other components of the cell as well as mitochondrial structure and function are more preserved in permeabilized fibers29,31,36, which makes this approach more physiologically relevant. With this protocol, mitochondrial functions can be accurately evaluated using high-resolution respirometry in only three thoraxes of Drosophila, with substrates allowing the determination of oxygen consumption at several different steps of the ETS. Therefore, this protocol could help answer key questions about the fundamental mechanisms that control metabolism in the context of many environmental or pathophysiological conditions by taking advantage of the Drosophila model.
To measure the oxygen consumption at several different steps of the ETS and evaluate how different substrates contribute to the respiration, different substrates (Figure 1), uncoupler, and inhibitors are used30 after permeabilization of the tissue. Specifically, sequential additions of different substrates are performed to stimulate the entry of electrons through different complexes of the ETS. An uncoupler, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), is then added at optimal concentration to measure the non-coupled respiration, i.e., the non-phosphorylating respiration stimulated to maximal oxygen consumption. Sequential inhibitions of complexes I, II, and III are then performed to monitor the residual oxygen consumption that is due to non-ETS oxidation reactions. Finally, complex IV maximal respiration capacity can be evaluated by the injection of N,N,N',N,-Tetramethyl-p-phenylenediamine (TMPD), an artificial electron provider, and ascorbate. It is important to note that the experiments are conducted at 24 °C since it is the temperature at which the flies are raised.
Protocol
1. Reagents Preparation
- Prepare the following solutions for dissection and permeabilization of the tissue.
- Prepare preservation solution: 2.77 mM CaK2EGTA, 7.23 mM K2EGTA, 5.77 mM Na2ATP, 6.56 mM MgCl2, 20 mM taurine, 15 mM Na2phosphocreatine, 20 mM imidazole, 0.5 mM dithiothreitol, and 50 mM K-MES, pH 7.1 (can be stored at -20 °C).
- Prepare saponin solution: 5 mg of saponin in 1 mL of preservation solution (prepare fresh daily).
- Prepare the following solutions for the measurement of the respiration.
- Prepare respiration medium: 120 mM KCl, 5 mM KH2PO4, 3 mM HEPES, 1 mM EGTA, 1 mM MgCl2, and 0.2% BSA (w/v), pH 7.2.
- Prepare substrates, uncoupler, inhibitors. Dissolve all substrates in H2O, and dissolve the uncoupler FCCP as well as the inhibitors in ethanol absolute (except malonate which is diluted in H2O). Neutralize most of the substrates as well as malonate (see Table of Materials). All concentrations given in the protocol are final concentrations inside 2 mL chambers.
2. Preparation and Calibration of the High-Resolution Respirometer.
- Wash the chambers, caps, and stoppers of the respirometer three times with 70% ethanol and three times with distilled water.
- Calibrate at zero oxygen concentration: pipet 2.3 mL of respiration medium in the chambers and add 10 mg of sodium dithionite. Although the maximal volume of the chamber is 2 mL, 2.3 mL are added to ensure that no air bubble stays under the stopper when closing the chambers. Close the chambers with the stoppers and measure the oxygen concentration for 10-20 min. Calibrate the electrodes at zero oxygen concentration with the resulting oxygen consumption rate.
- Wash the chambers with distillated water, incubate in absolute ethanol for 10 min, and then rinse with distilled water.
- Calibrate at air saturation: pipet 2.3 mL of respiration medium in the chambers, insert the stoppers, aspirate the excess medium on top of the stoppers, and then lift the stoppers with the spacer. Excess respiration medium is pipetted in the chambers (volume of 2 mL total) to avoid the presence of air bubbles after closing the chambers.
- Monitor the oxygen consumption for 45 min to an hour, until the oxygen concentration (blue trace) is stable around 250 nM at 24 °C.
3. Dissection of Flies and Permeabilization of Tissue
- Make sure all steps are performed on ice.
- Anaesthetize six male flies of the same strain, same age and raised on the same diet on ice to facilitate dissection of the flies (wild-type w1118, 15 days old, fed on cornmeal medium).
- Using a scalpel and fine-tipped forceps, dissect the flies (remove the heads and abdomens) to keep only the thoraxes containing the flight muscles. This step can be done with the naked eye. Handle the resulting thoraxes with fine-tipped forceps.
- Transfer three dissected thoraxes in a 25 mm Petri dish containing 2 mL of ice-cold preservation solution using the forceps.
- With the fine-tipped forceps, mechanically permeabilize the thoraxes by inserting the tip of the forceps into the thoraxes and repeatedly tear apart the tissue to obtain a loosely connected network.
- In a 24-well plate, fill two wells with 12.5 µL of the saponin solution and 1 mL of preservation solution to obtain a final concentration of 62.5 µg/mL of saponin. Fill the two adjacent wells with 1 mL of respiration medium.
- With the fine-tipped forceps, transfer three permeabilized thoraxes into the diluted saponin solution and incubate with mild agitation on an orbital shaker, on ice, for 20 min.
- After the latter incubation, transfer the fibers into the adjacent wells filled with the respiration medium with mild agitation, on ice, for 5 min to rinse off the saponin.
4. Determination of Dry Weight
- Dry the permeabilized thoraxes on an absorbent surface and flip 3-4 times using the fine-tipped forceps to make sure all moisture is removed.
- Weigh the three thoraxes together on a microbalance. Note the obtained weight as it will be used to normalize the mitochondrial oxygen consumption rates.
- Transfer the tissues immediately in a drop of respiration medium on ice.
5. Oxygen Consumption Rates Determination
- Open the chambers of the respirometer (remove the stoppers) and add 10 mM of pyruvate and 2 mM of malate into each chamber.
- Add the permeabilized tissues directly into the chambers filled with the respiration medium.
- Replace the stoppers using the spacer.
- With a 60 mL syringe, collect oxygen directly from an oxygen tank and inject 2-5 mL of oxygen through the stopper capillary of each chamber to make sure oxygen diffusion through the tissue will not limit oxygen consumption.
- Insert a Hamilton syringe into the capillary of each stopper to make sure the thoraxes remain in the chamber.
- Close the chambers completely when the oxygen concentration is around 400 nM, achieving oxygen levels above air saturation (around 160% air saturation) inside the chambers.
- Stop and restart the stirrers to check for air bubbles in the chambers.
- Enter the mass of tissue weighed previously to normalize respiration rates by mass of tissue (mg), displaying mass-specific oxygen consumption (pmol O2 consumed.s-1.mg-1 of tissue).
- Once the oxygen consumption is stabilized (red trace), add 5 mM ADP with a Hamilton syringe.
- After each injection, rinse the syringes with distilled water, 70% ethanol, and distilled water again.
- Once the oxygen consumption is stable again, inject 5 µL of a 4 mM of cytochrome c.
- Proceed to the sequential injections of the following substrates to evaluate the mitochondrial oxygen consumption at different steps of the ETS:
- Add 5 µL of a 2 M Proline.
- Add 10 µL of a 1 M succinate.
- Add 30 µL of a 1 M glycerol-3-phosphate.
- Inject the uncoupler FCCP in a titration manner by steps of 0.5-1 µM (2 µL of a 1 mM solution) until the optimal concentration is reached, i.e., the concentration to attain the highest oxygen consumption possible without inhibition (CAUTION: see Table of Materials).
- Inject the following inhibitors sequentially to entirely inhibit the electron flux in the ETS in order to measure the residual oxygen consumption (ROX) i.e. non-respiratory reactions such as oxygenase reactions among others:
- Add 1 µL of a 1 mM rotenone (CAUTION: see Table of Materials).
- Add 5 µL of a 2 M malonate (CAUTION: see Table of Materials).
- Add 1 µL of a 5 mM antimycin A (CAUTION: see Table of Materials).
- Add 0.2 mM ascorbate and 0.5 mM TMPD sequentially into the chambers, starting with the ascorbate to measure the oxygen consumption by complex IV.
- Add 20 mM of sodium azide to inhibit complex IV (CAUTION: see Table of Materials).
- Once the signal is stable, open the chambers with the spacer, re-oxygenate the chambers by injecting 2 mL of pure oxygen and close the chambers when the concentration is around 250-300 nmol.mL-1.
- Measure the signal for 10-15 min and calculate the chemical background, i.e., the oxygen consumption due to autoxidation of TMPD.
6. Cleaning of the Respirometer
- After the measurements, rinse the chambers with distilled water once and rinse the chambers three times in 70% ethanol.
- Incubate in absolute ethanol for 10 min to inactivate the inhibitors.
- Rinse three times with distilled water, fill the chambers with 70% ethanol and replace the stoppers and the caps until next utilization.
Representative Results
A representative trace of mitochondrial oxygen consumption using the protocol described above is provided in Figure 2. The pyruvate and malate injected in the chambers along with the permeabilized muscle fibers are referred to the CI-LEAK respiration, i.e., when the complex I of the ETS is stimulated by the NADH produced through oxidation of pyruvate and malate via the tricarboxylic acid cycle (CI). During this respiration rate, the mitochondrial oxygen is mainly maintained to compensate for the proton leak, i.e., protons crossing from the intermembrane space to the mitochondrial matrix, when ATP synthase is not active (LEAK)30. When the ADP is added, the ATP synthase is activated and the electrons are transferred from the complex I to the complex IV with concurrent phosphorylation of ADP into ATP (CI-OXPHOS), resulting in increased mitochondrial oxygen consumption. The OXPHOS coupling ratio is calculated as CI-OXPHOS/CI-LEAK and is usually taken as a good indicator of mitochondrial quality and of mitochondrial coupling30. In Drosophila, this ratio should at least exceed 6.0 (Figure 3). If it is below this value, it could indicate a problem with the tissue preparation or a mitochondrial dysfunction. The addition of cytochrome c allows the determination of the integrity of the outer mitochondrial membrane and is therefore used as a quality control of the preparation (Figure 4). Indeed, cytochrome c is loosely bound to the inner mitochondrial membrane and is typically washed away if the outer mitochondrial membrane is damaged during the permeabilization process. As a result, adding exogenous cytochrome c will significantly increase oxygen consumption if the outer mitochondrial membrane is damaged and the endogenous cytochrome c is lost. An increase of less than 10-15% in oxygen consumption (Figure 4) usually illustrates appropriate integrity of the outer mitochondrial membrane29. In Figure 3 and 4, the green traces were obtained from non-adequate permeabilization and handling of the samples (excessive tearing of the tissue for Figure 3, and weighed after a longer time on the absorbent surface for Figure 4), whereas the red traces represent samples adequately permeabilized and handled. These results highlight that appropriate permeabilization conditions are crucial for reliable assessment of mitochondrial oxygen consumption.
The following substrates used during the experiments provide electrons to the ubiquinone and allowed to increase the electron flux into the ETS. Proline is an amino acid that can be used as energy substrate notably in insects23, but also in mammals during acute starvation or during pathological conditions37. The addition of proline in the chambers allows to evaluate the contribution of proline dehydrogenase (ProDH) to the electron flux in the ETS, when electrons are flowing from both complex I and ProDH and are participating to the OXPHOS process (CI+ProDH-OXPHOS). With addition of succinate, the contribution of complex II (succinate dehydrogenase) to the ETS can be observed (CI+ProDH+CII-OXPHOS). Injection of glycerol-3-phosphate (G3P), further increases the mitochondrial oxygen consumption as this substrate stimulates the mitochondrial glycerol-3-phosphate dehydrogenase (G3PDH) which is part of the glycerophosphate shuttle and transfer electrons to the ETS (CI+ProDH+CII+G3PDH-OXPHOS). Both ProDH and G3PDH have been shown to be particularly active in several species of insects and are therefore important in this protocol with Drosophila20,21,22,23,32.
When the uncoupler FCCP is added, the non-coupled respiration (ETS state) is obtained, i.e., the maximal oxygen consumption representing the maximal capacity of the ETS (CI+ProDH+CII+G3P-ETS). The FCCP is a protonophore that transports the protons from the intermembrane space to the mitochondrial matrix without passing through complex V. The FCCP has to be carefully titrated, as concentrations below optimum result in non-maximal oxygen consumption and non-stable respiration rates while concentrations above optimum result in inhibition of mitochondrial oxygen consumption (Figure 5). Once the substrates and the uncoupler are added, inhibition of complexes I, II, and III can be sequentially performed using rotenone, malonate, and antimycin A, respectively. With each inhibitor used, a decrease in mitochondrial oxygen consumption is observed, until reaching the lowest oxygen consumption after the addition of antimycin A. The rate reached after the addition of all the inhibitors is the residual oxygen consumption30. It represents the oxygen consumption due to non-mitochondrial oxidative reactions such as oxygenase reactions and reactive species production for example, and has therefore to be subtracted from all the other rates measured.
TMPD is an artificial electron transporter that provides electrons directly to complex IV, bypassing all the complexes upstream, allowing measurement of the complex IV maximal respiration capacity. This substrate is however prone to autoxidation, so ascorbate has to be added prior to TMPD to limit but not completely avoid this autoxidation. To correct for the remaining autoxidation of TMPD, the Complex IV inhibitor sodium azide is added to the chambers and the oxygen consumption is then recorded for 10-15 minutes. This oxygen consumption therefore represents the chemical background, or in other words the autoxidation of TMPD that should be taken into account for the calculation of complex IV maximal respiration capacity.
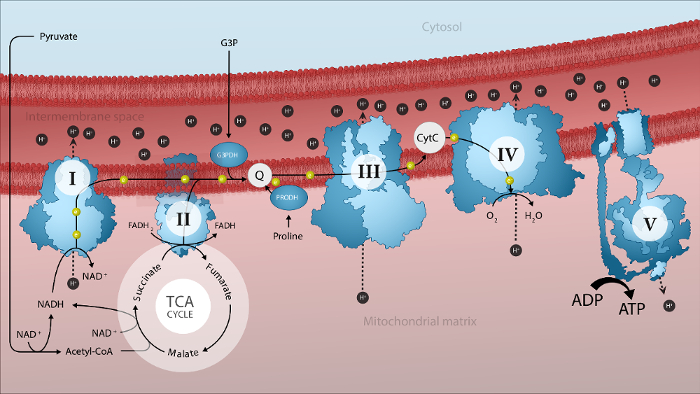
Figure 1. Schematic representation of mitochondrial electron transport by the electron transport system and oxidative phosphorylation in the mitochondrial inner membrane. I: complex I; II: complex II; III: complex III; IV: complex IV; V: ATP synthase; Acetyl-CoA: acetyl coenzyme A; Cyt c: cytochrome c; e–: electron; G3P: glycerol-3-phosphate; G3PDH: mitochondrial glycerol-3-phosphate dehydrogenase; H+: proton; PRODH: proline dehydrogenase; TCA: tricarboxylic acid cycle. Please click here to view a larger version of this figure.
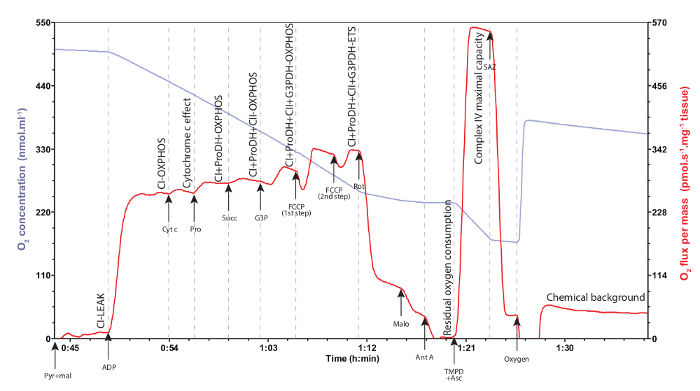
Figure 2: Representative traces of mitochondrial oxygen concentration (blue trace) and consumption (red trace) on permeabilized thoraxes of Drosophila. Each injection is represented with an arrow indicating the compound injected (Pyr: pyruvate; mal: malate; ADP; Cyt c: cytochrome c; Pro: proline; Succ: succinate; G3P: glycerol-3-phosphate; FCCP: carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; Rot: rotenone; Malo: malonate; Ant A: antimycin A; TMPD: N,N,N',N,-Tetramethyl-p-phenylenediamine; Asc: ascorbate; SAZ: sodium azide). Each mitochondrial oxygen consumption rate is denoted on the graph, when the oxygen consumption (red trace) has been stabilized. Cytochrome c effect denotes the integrity of the outer mitochondrial membrane (see text for details). Residual oxygen consumption represents the oxygen consumption due to oxidative side reactions in the cells and has to be subtracted to all the other rates measured. Chemical background denotes the oxygen consumption only due to autoxidation of TMPD and has to be taken into account for the calculation of the complex IV maximal respiration capacity. Please click here to view a larger version of this figure.
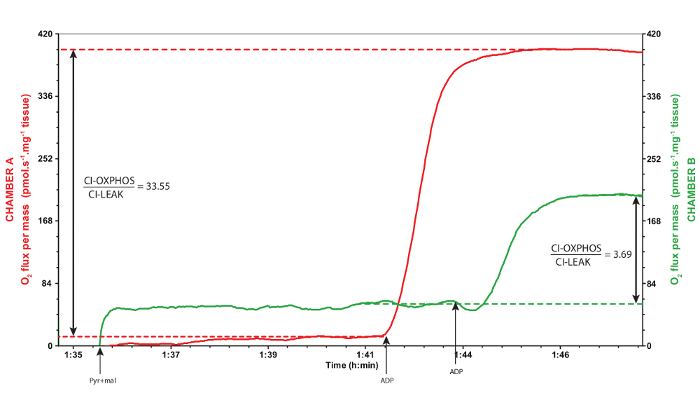
Figure 3: Representative traces of a valid (red trace, chamber A) and inadequate (green trace, chamber B) responses to the addition of ADP. The red trace corresponds to the chamber A and the green trace corresponds to the chamber B, during the same experiment. The red trace was obtained from thoraxes adequately permeabilized whereas the green trace was obtained after excessively tearing the tissues during the permeabilization. When ADP is added, an increase in the oxygen consumption is expected, like the red trace. OXPHOS coupling ratios are calculated as CI-OXPHOS/CI-LEAK and are presented in the graph. A ratio of less than 6.0 in Drosophila suggests a problem in the mitochondrial coupling and is characteristic of sample degradation or of mitochondrial dysfunction represented by the green trace. Pyr: pyruvate; mal: malate. Please click here to view a larger version of this figure.
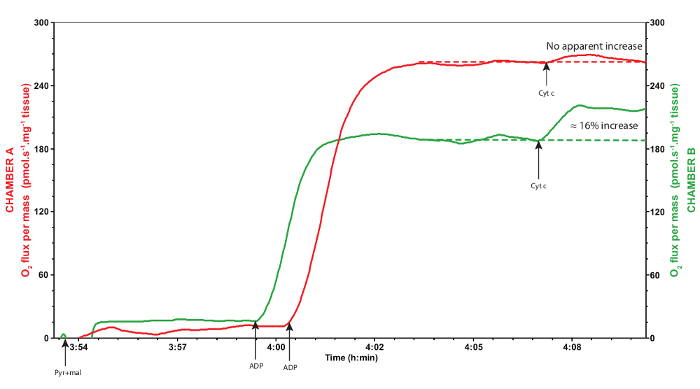
Figure 4: Representative traces of a valid (red trace, chamber A) and inadequate (green trace, chamber B) response to the addition of cytochrome c. The red trace corresponds to the chamber A and the green trace corresponds to the chamber B, during the same experiment. Red trace was obtained from thoraxes adequately permeabilized and handled, whereas the green trace was obtained after the thoraxes were purposely dried for a longer time on the absorbent surface before weighing. Cytochrome c does not usually increase mitochondrial oxygen consumption, as seen on the red trace, denoting that the outer mitochondrial membrane is intact. If, however, addition of exogenous cytochrome c significantly increases mitochondrial oxygen consumption of more than 15%, as seen on the green trace, it suggests that the outer mitochondrial membrane is damaged and hence that the sample is degraded and should be discarded. Pyr: pyruvate; mal: malate; Cyt c: cytochrome c. Please click here to view a larger version of this figure.
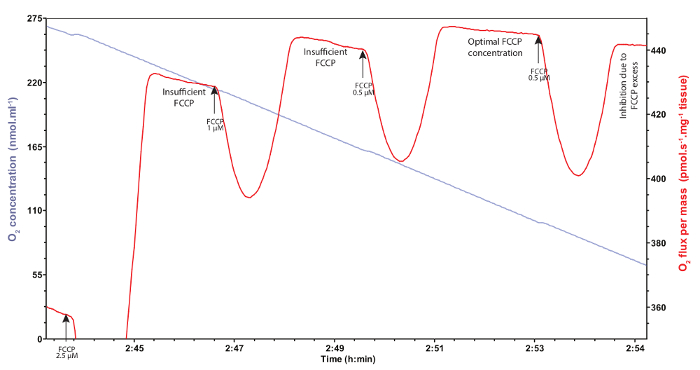
Figure 5: Representative traces of mitochondrial oxygen concentration (blue trace) and consumption (red trace) after titration of FCCP. Different injections of FCCP at several concentrations have to be carefully performed to determine the maximal oxygen consumption triggered by this uncoupler. Insufficient concentrations do not allow the assessment of maximal oxygen consumption and are characterized by non-stable respiration rates, whereas FCCP excess inhibits mitochondrial oxygen consumption. Arrows denote the different injections of FCCP. Please click here to view a larger version of this figure.
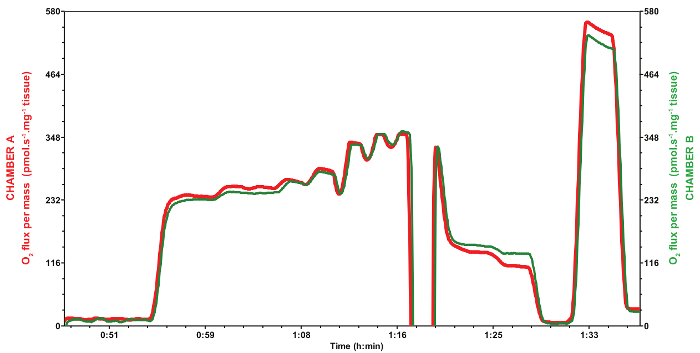
Figure 6: Reproducibility of mitochondrial oxygen consumption rates during a typical experiment obtained using the two different chambers (red trace, chamber A; green trace, chamber B) of a respirometer. The red trace corresponds to the chamber A and the green trace corresponds to the chamber B, during the same experiment using Drosophila from the same age, sex, strain and raised on the same diet, as well as the protocol described. Both samples were normalized with the mass of dry tissue weighed as described in the protocol (0.53 and 0.66 mg for chambers A and B, respectively). Please click here to view a larger version of this figure.
Discussion
In this study, a method for sample preparation prior to the measurements of mitochondrial oxygen consumption in Drosophila is described. This method was developed to overcome different problems related to the protocols using mitochondrial isolations, notably in terms of duration and number of individuals required. Instead of working with mitochondrial isolations usually requiring large amount of tissues obtained from several individuals, this experiment is performed on permeabilized muscle fibers from thoraxes of few Drosophila. In this protocol, only three individuals are needed to display optimal results. Therefore, the measurement of mitochondrial oxygen consumption at various time points is possible as it is more achievable to synchronize a smaller group of flies having the same age and of the same sex.
First, it is important to use Drosophila of the same age, sex, strain and raised in the same conditions to obtain low variability in the results (Figure 6), as respiration can change with the age, the gender, the genotype, and the diet of the flies. The most critical step in the execution of this protocol is the permeabilization procedure of the thoraxes. It is very important to proceed quickly to avoid the degradation of tissue, and it is also important to make sure that the thoraxes are well permeabilized to have the maximal rates of oxygen consumption. To ensure that the permeabilization has been performed correctly and that the structural and functional integrity of the mitochondria are preserved after permeabilization, the OXPHOS coupling ratio (CI-OXPHOS/CI-LEAK, Figure 3), as well as the response to cytochrome c (Figure 4) are used as quality controls. There is a tradeoff to take into consideration between working quickly and making sure the permeabilization is well executed. It is also critical to always work on ice when manipulating the dissected thoraxes. To obtain the most reproducible results (Figure 6), homogenous permeabilization is necessary and practicing the technique for the permeabilization helps to make sure that the same movements are repeated. Another critical step is the determination of the dry weight of the sample. Indeed, after the thoraxes are incubated, they are very sensitive, fragile and prone to degradation. Therefore, similarly to the permeabilization, quickly proceeding to the determination of dry weight is important. On the other hand, it is important to determine the weight with accuracy as the results are normalized by the mass of the tissue. The quantity of thoraxes used can also be optimized. The protocol can be adapted to use a single thorax per chamber35, but a certain level of resolution could be lost in the measurement of oxygen consumption as much as in the determination of the dry weight. The quantity of thoraxes can be increased if necessary, but it is important to note that the more mitochondria in the sample, the higher the oxygen consumption and the chambers will have therefore to be reoxygenated. This reoxygenation can be done by opening the chambers and inject oxygen, but it will increase the probability to insert air bubbles in the chamber. Therefore, if possible, try to avoid having to reoxygenate the chambers (except when complex IV maximal respiration capacity has to be determined). Before running the experiments, optimization of the substrate concentrations is also necessary to attain the highest mitochondrial oxygen consumption without observing inhibitory effects due to an excessive concentration. The chambers of the respirometer also have to be thoroughly cleaned in order to avoid potential contaminations. It is important to start the cleaning with distillated water and then 70% ethanol to get rid of potential biological contaminations. Following this, an incubation in absolute ethanol for 10 min is recommended to avoid contamination of hydrophobic inhibitors. Finally, rinsing the chambers with distillated water and filling them with 70% ethanol will avoid potential contaminations until the next utilization.
The protocol can be easily modified to study the effect of either other substrates, or different inhibitors. For example, an interesting protein to study is the mitochondrial pyruvate carrier (MPC). The role of this protein is to transport pyruvate generated in the cytosol into the mitochondrial matrix. Recently, it has been suggested to play an important role in the modulation of metabolism during pathological conditions such as during type 2 diabetes38. To study the effect of the protein MPC on mitochondrial metabolism, it is useful to start the injections with pyruvate only instead of pyruvate and malate at the same time. Thus, the effect of the pyruvate is studied independently from the effect of malate, as the latter is injected to only support the tricarboxylic acid cycle and ensure that intermediates are not depleted. It is also possible to use inhibitors of MPC39 to evaluate if a limitation of this transporter rather than the oxidation of pyruvate is observed. Then, it would be possible to observe a potential shift in the preferred fuel source for respiration40. It is also possible to omit some substrates to simplify the protocol if it is not relevant for the study. For example, to study a dysfunction of the complex I only, it is not necessary to use all the substrates presented in this protocol.
This protocol presents some limitations that divert from in vivo conditions. The measurements of mitochondrial oxygen consumption are recorded when the respiration medium is above air-saturation, as pure oxygen is injected to prevent limitation of oxygen diffusion inside the tissue30. Moreover, the concentrations of substrates are injected in excess, which are not reflecting the in vivo conditions. It is also important to note that the age, sex, strain and diet of Drosophila can affect the results. These parameters are therefore to be taken into account when interpreting the results. This method is however closer to physiological conditions than methods using mitochondrial isolations to measure mitochondrial oxygen consumption, as the interactions with the other cellular components and the structural integrity of mitochondria and morphology are conserved31. It is important to note that mitochondrial isolations are more advantageous when additional parameters such as ROS production or mitochondrial efficiency (P/O ratio) have to be measured27.
This method can be used to achieve various goals, ranging from the understanding of mitochondrial dysfunctions and the underlying mechanisms of metabolic diseases, to testing the effects of different compounds on mitochondrial functions under pathological conditions. Moreover, it can be used to observe the effects of several environmental stresses on the mitochondrial metabolism, such as changes in diet or temperature. For example, it is possible to study the efficiency of bioactive synthetic molecules on the mitochondrial functions and in the treatment of mitochondrial dysfunctions. This method is also a good tool to study the mitochondrial functions at different time points, which makes it very relevant for the study of the fundamental mechanisms related to the aging process. The assessment of mitochondrial metabolism via oxygen consumption using permeabilized thoraxes will certainly help to better our knowledge about the role of mitochondrial metabolism when the cell is exposed to challenging conditions.
開示
The authors have nothing to disclose.
Acknowledgements
This study was funded by grants from the National Sciences and Engineering Research Council (NSERC, discovery grant) and Université de Moncton to NP. LHB would like to acknowledge the funding support from the Canadian Institute of Health Research (CIHR), the New Brunswick Innovation Foundation (NBIF) and Université de Moncton. The work of EHC is supported by the Alzheimer Society of Canada, Brain Canada, NSERC, Canadian Breast Cancer Foundation, New Brunswick Innovation Foundation, New Brunswick Health Research Foundation and Université de Moncton.
Materials
| High-resolution respirometer Oxygraph O2K | Oroboros Instruments, Innsbruck, Austria | 10022-02 | Startup O2K respirometer kit |
| O2K-Titration Set | Oroboros Instruments, Innsbruck, Austria | 20820-03 | Hamilton syringes with different volumes |
| Datlab software | Oroboros Instruments, Innsbruck, Austria | 20700 | Software for data acquisition and analysis |
| Fine-tipped antimagnetic forceps | VWR | 82027-400 | |
| Secura225D-1S-DQE | Sartorius AG, Goettingen, Germany | Semi-micro balance (distributed by several companies) |
|
| Drosophila melanogaster wild-type w1118 | Bloomington Drosophila stock Center, IN, USA | Storage Condition: 24 °C |
|
| Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid | Sigma-Aldrich | E4378 | EGTA Storage Condition: RT |
| KOH | Sigma-Aldrich | P1767 | CAUTION: corrosive to metals, acute toxicity, skin corrosion, serious eye damage, acute aquatic toxicity. Storage Condition: RT |
| CaCO3 | Sigma-Aldrich | C4830 | Storage Condition: RT |
| Na2ATP | Sigma-Aldrich | A2383 | Storage Condition: -20 °C |
| MgCl2.6H2O | Sigma-Aldrich | M9272 | Storage Condition: RT |
| Taurine | Sigma-Aldrich | T0625 | Storage Condition: RT |
| Na2Phosphocreatine | Sigma-Aldrich | P7936 | Storage Condition: -20 °C |
| Imidazole | Sigma-Aldrich | I5513 | Storage Condition: RT |
| Dithiothreitol | Sigma-Aldrich | D0632 | Storage Condition: 2-8 °C |
| MES hydrate | Sigma-Aldrich | M8250 | Storage Condition: RT |
| Saponin from quillaja bark | Sigma-Aldrich | S7900 | Saponin Storage Condition: RT Solution Preparation: 5 mg in 1 mL of preservation solution. Prepare fresh daily. |
| KCl | Sigma-Aldrich | P9541 | Storage Condition: RT |
| KH2PO4 | Sigma-Aldrich | P9791 | Storage Condition: RT |
| HEPES | Sigma-Aldrich | H3375 | Storage Condition: RT |
| BSA | Sigma-Aldrich | 05470 | Storage Condition: 2-8 °C |
| Na2S2O4 | Sigma-Aldrich | 157953 | Sodium dithionite. CAUTION: self-heating substances and mixtures, acute toxicity, acute aquatic toxi chronic aquatic toxicity. Storage Condition: RT |
| Sodium pyruvate | Sigma-Aldrich | P2256 | Pyruvate Storage Condition: 2-8 °C Solution Preparation: In MilliQ water. Prepare fresh daily. |
| L-(-)-Malic acid | Sigma-Aldrich | M1000 | Malate Storage Condition: RT Solution Preparation: In MilliQ water. Neutralize with KOH and store at -20 °C. |
| Adenosine 5'-diphosphate monopotassium salt hydrate | Sigma-Aldrich | A5285 | ADP Storage Condition: -20 °C Solution Preparation: In MilliQ water. Neutralize with KOH and store at -80 °C. |
| Cytochrome c from equine heart | Sigma-Aldrich | C7752 | Cytochrome c Storage Condition: -20 °C Solution Preparation: In MilliQ water. Store at -20 °C. |
| L-Proline | Sigma-Aldrich | P0380 | Proline Storage Condition: RT Solution Preparation: In MilliQ water. Store at -20 °C. |
| Sodium succinate dibasic hexahydrate | Sigma-Aldrich | S2378 | Succinate Storage Condition: RT Solution Preparation: In MilliQ water. Neutralize with HCl and store at -20 °C. |
| sn-Glycerol 3-phosphate bis(cyclohexylammonium) salt | Sigma-Aldrich | G7886 | Glycerol-3-phosphate Storage Condition: -20 °C Solution Preparation: In MilliQ water. Neutralize with HCl and store at -80 °C. |
| Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone | Sigma-Aldrich | C2920 | FCCP. CAUTION: acute toxicity, skin sensitisation, chronic aquatic toxicity. Storage Condition: RT Solution Preparation: In absolute ethanol. Store in glass vials at -20 °C. |
| Rotenone | Sigma-Aldrich | R8875 | CAUTION: acute toxicity, skin irritation, eye irritation, specific target organ toxicity (respir sytem), acute aquatic toxicity, chronic aquatic toxicity. Solution Preparation: In absolute ethanol. Store in dark vials at -20 °C. |
| Malonic acid | Sigma-Aldrich | M1296 | Malonate. CAUTION: acute toxicity, serious eye damage. Storage Condition: RT Solution Preparation: In MilliQ water. Neutralize with KOH. Prepare fresh daily. |
| Antimycin A from Streptomyces sp. | Sigma-Aldrich | A8674 | Antimycin A. CAUTION: acute toxicity, acute aquatic toxicity, chronic aquatic toxicity. Storage Condition: -20 °C Solution Preparation: In absolute ethanol. Store at -20 °C. |
| N,N,N′,N′-Tetramethyl-p-phenylenediamine | Sigma-Aldrich | T7394 | TMPD Storage Condition: RT Solution Preparation: In MilliQ water. Store in dark vials at -20 °C. |
| (+)-Sodium L-ascorbate | Sigma-Aldrich | A4034 | Ascorbate Storage Condition: RT Solution Preparation: In MilliQ water. Store in dark vials at -20 °C. |
| NaN3 | Sigma-Aldrich | S2002 | Sodium azide. CAUTION: acute toxicity (oral and dermal), specific target organ toxicity (brain), aquatic toxicity, chronic aquatic toxicity. Solution Preparation: In MilliQ water. Store at -20 °C. |
参考文献
- Stephenson, R., Metcalfe, N. H. Drosophila melanogaster: a fly through its history and current use. The journal of the Royal College of Physicians of Edinburgh. 43 (1), 70-75 (2013).
- Morgan, T. H. An attempt to analyze the constitution of the chromosomes on the basis of sex-limited inheritance in Drosophila. Journal of Experimental Zoology. 11 (4), 365-413 (1911).
- Dobzhansky, T., Wright, S. Genetics of Natural Populations. V. Relations between Mutation Rate and Accumulation of Lethals in Populations of Drosophila Pseudoobscura. 遺伝学. 26 (1), 23-51 (1941).
- Zipursky, S. L., Rubin, G. M. Determination of Neuronal Cell Fate: Lessons from the R7 Neuron of Drosophila. Annual Review of Neuroscience. 17 (1), 373-397 (1994).
- Costa, R., Speretta, E., Crowther, D. C., Cardoso, I. Testing the therapeutic potential of doxycycline in a Drosophila melanogaster model of Alzheimer disease. The Journal of biological chemistry. 286 (48), 41647-41655 (2011).
- Blandini, F., Armentero, M. T. Animal models of Parkinson’s disease. FEBS Journal. 279 (7), 1156-1166 (2012).
- Baker, K. D., Thummel, C. S. Diabetic Larvae and Obese Flies-Emerging Studies of Metabolism in Drosophila. Cell Metabolism. 6 (4), 257-266 (2007).
- Morris, S. N. S., et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochimica et Biophysica Acta – Molecular Basis of Disease. 1822 (8), 1230-1237 (2012).
- Abou-Sleiman, P. M., Muqit, M. M. K., Wood, N. W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nature Reviews Neuroscience. 7 (3), 207-219 (2006).
- McGurk, L., Berson, A., Bonini, N. M. Drosophila as an in vivo model for human neurodegenerative disease. 遺伝学. 201 (2), 377-402 (2015).
- Lin, M. T., Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 443 (7113), 787-795 (2006).
- Carri, M. T., Valle, C., Bozzo, F., Cozzolino, M. Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS. Frontiers in Cellular Neuroscience. 9, 1-6 (2015).
- Balaban, R. S., Nemoto, S., Finkel, T. Mitochondria, oxidants, and aging. Cell. 120 (4), 483-495 (2005).
- Szibor, M., Holtz, J. Mitochondrial ageing. Basic Research in Cardiology. 98 (4), 210-218 (2003).
- Palikaras, K., Lionaki, E., Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 521 (7553), 525-528 (2015).
- López-Lluch, G., Irusta, P. M., Navas, P., de Cabo, R. Mitochondrial biogenesis and healthy aging. Experimental Gerontology. 43 (9), 813-819 (2008).
- Muoio, D. M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell. 159 (6), 1253-1262 (2014).
- Efeyan, A., Comb, W. C., Sabatini, D. M. Nutrient-sensing mechanisms and pathways. Nature. 517 (7534), 302-310 (2015).
- Brown, G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. The Biochemical journal. 284 (1), 1-13 (1992).
- McDonald, A. E., Pichaud, N., Darveau, C. A. "Alternative" fuels contributing to mitochondrial electron transport: Importance of non-classical pathways in the diversity of animal metabolism. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. , (2017).
- Soares, J. B. R. C., Gaviraghi, A., Oliveira, M. F., Veuthey, J., Zamboni, N., Westermann, B. Mitochondrial Physiology in the Major Arbovirus Vector Aedes aegypti: Substrate Preferences and Sexual Differences Define Respiratory Capacity and Superoxide Production. PLOS ONE. 10 (3), e0120600 (2015).
- Newell, C., Kane, C. L., Kane, D. A. Mitochondrial substrate specificity in beetle flight muscle: assessing respiratory oxygen flux in small samples from Dermestes maculatus and Tenebrio molitor. Physiological Entomology. 41 (2), 96-102 (2016).
- Teulier, L., Weber, J. M., Crevier, J., Darveau, C. A. Proline as a fuel for insect flight: enhancing carbohydrate oxidation in hymenopterans. Proceedings of the Royal Society of London B: Biological Sciences. 283 (1834), 20160333 (2016).
- Ferguson, M., Mockett, R. J., Shen, Y., Orr, W. C., Sohal, R. S. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. The Biochemical journal. 390 (2), 501-511 (2005).
- Miwa, S., Brand, M. D. The topology of superoxide production by complex III and glycerol 3-phosphate dehydrogenase in Drosophila mitochondria. Biochimica et Biophysica Acta (BBA) – Bioenergetics. 1709 (3), 214-219 (2005).
- Katewa, S. D., Ballard, J. W. O. Sympatric Drosophila simulans flies with distinct mtDNA show difference in mitochondrial respiration and electron transport. Insect Biochemistry and Molecular Biology. 37 (3), 213-222 (2007).
- Brand, M. D., Nicholls, D. G. Assessing mitochondrial dysfunction in cells. Biochemical Journal. 435 (2), 297-312 (2011).
- St-Pierre, J., Buckingham, J. A., Roebuck, S. J., Brand, M. D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. Journal of Biological Chemistry. 277 (47), 44784-44790 (2002).
- Kuznetsov, A. V., Veksler, V., Gellerich, F. N., Saks, V., Margreiter, R., Kunz, W. S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nature Protocols. 3 (6), 965-976 (2008).
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. International Journal of Biochemistry and Cell Biology. 41 (10), 1837-1845 (2009).
- Picard, M., Taivassalo, T., Gouspillou, G., Hepple, R. T. Mitochondria: isolation, structure and function. The Journal of Physiology. 589 (18), 4413-4421 (2011).
- Pichaud, N., Ballard, J. W. O., Tanguay, R. M., Blier, P. U. Thermal sensitivity of mitochondrial functions in permeabilized muscle fibers from two populations of Drosophila simulans with divergent mitotypes. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 301 (1), R48-R59 (2011).
- Pichaud, N., Ballard, J. W. O., Tanguay, R. M., Blier, P. U. Naturally occurring mitochondrial dna haplotypes exhibit metabolic differences: insight into functional properties of mitochondria. Evolution. 66 (10), 3189-3197 (2012).
- Pichaud, N., Messmer, M., Correa, C. C., Ballard, J. W. O. Diet influences the intake target and mitochondrial functions of Drosophila melanogaster males. Mitochondrion. 13 (6), 817-822 (2013).
- Wolff, J. N., Pichaud, N., Camus, M. F., Côté, G., Blier, P. U., Dowling, D. K. Evolutionary implications of mitochondrial genetic variation: mitochondrial genetic effects on OXPHOS respiration and mitochondrial quantity change with age and sex in fruit flies. Journal of Evolutionary Biology. 29 (4), 736-747 (2016).
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. The International Journal of Biochemistry & Cell Biology. 41 (10), 1837-1845 (2009).
- Phang, J. M., Donald, S. P., Pandhare, J., Liu, Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 35 (4), 681-690 (2008).
- Bender, T., Martinou, J. C. The mitochondrial pyruvate carrier in health and disease: To carry or not to carry?. Biochimica et Biophysica Acta – Molecular Cell Research. 1863 (10), 2436-2442 (2016).
- Divakaruni, A. S., et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 110 (14), 5422-5427 (2013).
- McCommis, K., et al. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Molecular Metabolism. 5 (8), 602-614 (2016).

