Bioprinting of Cartilage and Skin Tissue Analogs Utilizing a Novel Passive Mixing Unit Technique for Bioink Precellularization
概要
Cartilage and skin analogs were bioprinted using a nanocellulose-alginate based bioink. The bioinks were cellularized prior to printing via a single step passive mixing unit. The constructs were demonstrated to be uniformly cellularized, have high viability, and exhibit favorable markers of differentiation.
Abstract
Bioprinting is a powerful technique for the rapid and reproducible fabrication of constructs for tissue engineering applications. In this study, both cartilage and skin analogs were fabricated after bioink pre-cellularization utilizing a novel passive mixing unit technique. This technique was developed with the aim to simplify the steps involved in the mixing of a cell suspension into a highly viscous bioink. The resolution of filaments deposited through bioprinting necessitates the assurance of uniformity in cell distribution prior to printing to avoid the deposition of regions without cells or retention of large cell clumps that can clog the needle. We demonstrate the ability to rapidly blend a cell suspension with a bioink prior to bioprinting of both cartilage and skin analogs. Both tissue analogs could be cultured for up to 4 weeks. Histological analysis demonstrated both cell viability and deposition of tissue specific extracellular matrix (ECM) markers such as glycosaminoglycans (GAGs) and collagen I respectively.
Introduction
In recent years, three-dimensional (3D) bioprinting technology has become more accessible to researchers, allowing the technique to become more widely utilized for fabrication of tissue analogs. Bioprinting promises to revolutionize biomedical research by facilitating the rapid and repeatable fabrication of multifaceted tissue constructs. The crux of the bioprinting technology lays in the ability to precisely control the deposition of biomaterials (known as bioinks) in three dimensions. This allows the generation of complex scaffolds with distinct regions of matrix compositions, bioactive factors, and cells that can more accurately recapitulate native tissue structure.
Bioprinting has been utilized for the fabrication of constructs for many tissue applications including cartilage1, skin2, muscle3, and bone4. These tissues are attractive for bioprinting due to their intrinsic striated micro-architectures that are suitable for recapitulation via layer-by-layer deposition. In particular, skin possesses a well-defined multilayered structure5, which is suitable for fabrication through layer-by-layer deposition techniques such as bioprinting. Additionally, bioprinting can be utilized to generate constructs that possess the necessary anatomical dimensions and shapes to repair the tissue defect. The ability to generate biomaterials with patient-specific size and shape6 can begin to address the demand for partial repairs of many tissues including but not limited to bone defects, cartilage damage, and skin lesions whose extent varies from patient-to-patient.
In this study, two tissue analogs (articular cartilage and skin) were fabricated through the bioprinting of pre-cellularized bioinks. Ensuring adequate blending of a bioink with cell suspension that can ensure uniform cell distribution while preserving cell viability can be a challenge. Bioinks suitable for bioprinting via extrusion are often highly viscous and therefore require extensive mixing to ensure a homogenous blend. Mechanical damage to cells can occur under harsh mixing conditions and negatively affect viability. Studies have shown that most cell death during the inkjet printing process occurs during preparation such as mixing7,8. While traditional mixing with agitation9 may be sufficient for low viscosity bioinks suitable for inkjet printing10, mixing of cells into a high viscosity bioink more suitable for extrusion bioprinting is more difficult. Addressing this need, the use of mixing nozzles has become more popular for the blending of bioinks during the printing process11. These mixers have also been widely utilized in microfluidics research where the mixing of fluids with low Reynolds number is important12. The utilization of a continuous mixing process to blend a cell suspension into a bioink would allow for uniformity during the printing process. However, since cell suspensions possess low viscosity compared to a bioink, difficulties will arise in preventing sedimentation of the cells during the printing process9,13,14. Alternatively, the mixing of cells into a bioink prior to printing may address this issue.
To minimize cell death during blending into a bioink, we developed a technique based on a passive mixing unit to blend cells into a bioink in the minimal number of steps. The chaotic mixing generated through the flow of the materials through the mixing unit is sufficient to reproducibly blend two components together15,16. This method was primarily developed to simplify the blending of any cell suspension with any bioink that suitable for extrusion bioprinting. The number of steps in the mixing process was minimized to eliminate user-to-user variation in mixing. Excessive mixing steps can be time consuming and not applicable to all bioinks, particularly when cells are involved. Secondary, we aimed to develop a mixing process that was self-contained to both preserve sterility and minimize sample loss.
In this manuscript, we demonstrate the blending of a cell suspension with a bioink using a passive mixing unit technique that minimizes handling and results in high cell viability and uniform distribution. These pre-cellularized bioinks are then utilized to bioprint either a cartilage or skin construct with one or two cell types, respectively that are cultured for up to 6 weeks. The bioink utilized is an alginate-nanocellulose blend, which previously has shown suitability for bioprinting1.
Protocol
This protocol follows the guidelines of Chalmers University's human research ethics committee.
NOTE: All steps are to be performed within a sterile biosafety cabinet.
1. Preparation of Consumables, Bioink, and Cells
- Obtain two syringes, one syringe (Figure 1a) is for the cell suspension, while the other syringe is for the bioink (Figure 1b).
- Obtain a sterile passive mixing unit (Figure 1c) that can be coupled with dual syringes via a Luer lock connection.
- Obtain a dispensing unit (Figure 1d) that can extrude a volume from two sterile syringes simultaneously at a controlled rate.
NOTE: The mixing ratio utilized in this study is 10:1. Therefore, use a 12 mL syringe and a 1 mL syringe as required by the 10:1 mixing unit. - Obtain a sterile cartridge (Figure 1e) and a sterile female-female Luer lock connector to directly mixed the bioink and cell suspension into.
- Obtain or prepare the bioink for blending with a cell suspension.
NOTE: In this protocol, a nanocellulose/alginate based bioink was utilized. This bioink is cross-linked through the addition of a sterile 100 mM CaCl2 solution post printing. - Detach cells using a 0.5% trypsin/EDTA solution and count the total number of cells using the trypan blue exclusion method17.
NOTE: In this protocol, human fibroblasts were utilized. - Determine what cell density is desired in the final printed construct. Calculate using the following equations the concentration of the harvested cells that must be diluted to achieve this target final cell density.
NOTE: In this protocol, a final cell density of 5 x 106 cells/mL was utilized.- Select a desired cell concentration for the experiments: Ccell (cells/mL).
- Calculate the amount of bioink necessary, Vbioink, based on the total number of constructs desired:
Vbioink = Vconstruct × NContructs
Note: For example, the volume of bioink per construct is 100 µL. If 30 constructs are printed, then the bioink volume needed is 3 mL. - Calculate the number of cells needed:
Ncells = Ccell (1.1 × Vbiolink) - Resuspend the cells (Ncells) in 1/10th the volume of the bioink:
Vcell suspension = 0.1 × Vbiolink
NOTE: For example, if Vbioink = 3 mL, then Vcell suspension = 0.1 × 3 mL = 0.3 mL
2. Mixing of Cell Suspension and Bioink
- Transfer the cell suspension into the cell suspension syringe.
- Transfer the bioink to another syringe or obtain a syringe containing the bioink.
- Pull the bioink syringe plunger back and insert the syringe into the dispensing unit. Position the unit vertically with the Luer lock connector upwards (Figure 1f1).
- Pull the plunger of the cell syringe back to a similar length as the bioink syringe and insert into the dispensing unit (Figure 1f2).
- Attach both syringes to the mixing unit by twisting the Luer lock connectors (Figure 1f3).
- Prime the mixing system by pushing on the dispensing unit to extrude the air in the syringe. Stop the priming prior to the solution reaching the Luer lock (Figure 1g4).
- After priming, attach the filling cartridge to the end of the mixing unit via the Luer lock connector (Figure 1g5). Ensure that the plunger in the filling cartridge is at the bottom prior to attachment.
- Slowly compress (Figure 1h6) the dispensing unit to mix the bioink and cell suspension together into the cartridge (Figure 1i7).
- Push the plunger in the filling cartridge downward with a sterile pipet tip to contact the bioink-cell mixture after mixing. Keep the dispensing until compressed to ensure the cell/bioink mixture is not extruded back into the mixing unit.
- Cap the cartridge and gently tap on the work surface to move any air bubbles to the top of the cartridge (piston end).
NOTE: At this point, the cell/bioink mixture is ready for printing. The following sections will outline specific applications and printing procedures.
3. Determination of Cell Viability Using a Mixing Unit Compared to Manual Spatula Mixing
- Detach human fibroblasts (passage 7) with a 0.5% trypsin/EDTA solution at 80% confluence, count the number, and resuspend in culture medium at sufficient cell density to achieve a final concentration after blending with the bioink (1:10 cell:bioink ratio) of 5 x 106 cells/mL.
- Blend cells into the bioink using either the passive mixing unit technique (Step 2) or via spatula to evaluate the effect of both techniques on cell viability.
- Blend the cells into the bioink using the passive mixing unit technique 1, 2, or 3 times prior to dispensing into a mold for cross-linking using 100 mM CaCl2.
NOTE: To perform additional blends, mix the cell/bioink directly into a syringe rather than a cartridge. Then remix the blend through the mixing unit following the previous protocol but without the cell syringe component. - Blend the cells into a separate bioink using manual mechanical mixing via a spatula for durations of 30, 60, or 90 s. Transfer the mixtures (for each mixing time) into a mold for cross-linking using 100 mM CaCl2.
- Transfer the samples to a well plate after the completion of cross-linking and culture under standard conditions.
- After 1 day of culture, wash the constructs (n = 3 – 4 per group) in serum-free cell culture medium for 30 min. Stain the cells in the constructs with a staining solution (4 µM Calcein AM, 1 µM Ethidium homodimer-1) for 30 min.
- Wash two additional times, and incubate the samples in serum-free cell culture medium for a total of 1 h at 37 °C. Transfer the samples to a live cell imaging solution.
- Acquire images via a digital color camera at 10X magnification using an inverted microscope with FITC and Texas Red filters, and analyze using image analysis software.
- Randomly select three images from each construct for quantification of cell viability.
- Calculate viability based on the ratio of live cells to total number of cells. Analyze the data via a one-way ANOVA followed by Tukey's multiple comparisons test.
4. Bioprinting of Cartilage Analogs with a Single Cell Type
- Draw a 3D model of the desired tissue analog. Convert to a Gcode file for bioprinting and load the Gcode file on the bioprinter1.
NOTE: In this protocol, a square structure with dimensions 4.8 x 4.8 x 0.9 mm3 was exported as an STL file. A Gcode file was generated of the lattice structure using the following settings: layer thickness, 0.3 mm; infill pattern, rectilinear; infill density, 25%; speed, 10 mm/s. - Isolate and cryopreserve primary human nasal chondrocytes (hNC) from patients following the referenced protocol1.
- Thaw and expand cryopreserved hNCs and expand once in monolayer culture using standard culture medium at 37 °C. Detach cells at 80 – 90% confluence with a 0.5% trypsin/EDTA solution and count using a trypan blue exclusion protocol. All experiments were conducted using hNCs at passage 2.
- Resuspend the hNCs at 100 x 106 cells/mL within 300 µL of culture medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 50 µg/mL ascorbic acid, in preparation for blending with the bioink.
- Blend the hNC cell suspension into a nanocellulose/alginate based bioink following the passive mixing unit protocol at a 10:1 bioink:cell suspension ratio to obtain a final cell concentration of 9 x 106 cells/mL.
- Ensure that the bioprinter is sterilized via UV exposure and wipe down with 70% ethanol. Maintain sterility by placing in a laminar flow cabinet.
- Attach sterile printing nozzles to the cartridges containing the bioink/cell suspension blends and insert into the bioprinter.
- Calibrate the bioprinter either manually or by the protocols specific to the printer.
- Bioprint the lattice-structured, cell-laden constructs using the following printing parameters: 25G conical nozzle at a pressure of 25 kPa. Bioprint cell-free constructs (blended with cell medium containing no cells) as a control.
- Cross-link the constructs by adding an ionic solution of 100 mM CaCl2 for 5 min. Rinse the constructs and incubate in culture medium under standard culture conditions (37 °C, 5% CO2, and 95% relative humidity). Change the media every second or third day.
- Collect samples for histological analysis at weeks 2 and 4. Stain the samples for GAG production using an Alcian blue stain18.
5. Bioprinting of Skin Analogs with Two Cell Types
- Draw a 3D model of the desired tissue analog and convert to a Gcode file for bioprinting. Load the Gcode file on the bioprinter.
NOTE: In this protocol, a square structure with dimensions 4.8 x 4.8 x 0.9 mm3 was exported as an STL file. Then a Gcode file was generated of the lattice structure using the following settings: layer thickness, 0.3 mm; infill pattern, rectilinear; infill density, 25%; speed, 10 mm/s. - Prepare the cells for blending into the bioink for bioprinting. For the fabrication of skin analogs, two cell types were utilized. The two cell types were blended into the bioink and bioprinting.
- Obtain primary HDF. Maintain these cells in DMEM growth media supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 50 µg/mL ascorbic acid. Detach cells at 80 – 90% confluence with a 0.5% trypsin/EDTA solution and count.
- Isolate and cryopreserve primary hNC from patients following the referenced protocol1. Thaw and expand cryopreserved hNCs in monolayer culture. Detach cells at 80 – 90% confluence with a 0.5% trypsin/EDTA solution and count.
- Resuspend both cell types at 100 x 106 cells/mL within growth media. Mix the cell suspensions together at a 1:1 ratio to achieve a final total concentration of 100 x 106 cells/mL in 300 µL of media.
- Blend the 50:50 cell suspension of HDF and hNC into a nanocellulose/alginate based bioink following the passive mixing unit protocol at a 10:1 bioink:cell suspension ratio to obtain a final cell concentration of 9 x 106 cells/mL.
- Ensure that the bioprinter is sterilized or is placed in a laminar flow cabinet to maintain sterility.
- Attach sterile printing nozzles to the cartridges containing the bioink/cell suspension blends and insert into the bioprinter.
- Calibrate the bioprinter either manually or the protocols specific to the printer.
- Bioprint the lattice-structured, cell-laden constructs using the following printing parameters: 25G conical nozzle at a pressure of 25 kPa. Bioprint cell-free constructs (blended with cell medium containing no cells) as a control.
- Cross-link the constructs by adding an ionic solution of 100 mM CaCl2 for 5 min. Rinse the constructs and incubate in culture medium under standard culture conditions (37 °C, 5% CO2, and 95% relative humidity). Change the media every second or third day.
- Collect samples for histological analysis at weeks 2 and 4. Stain the samples for collagen I production using a Masson's trichrome stain19.
Representative Results
Results in this manuscript are divided into two sections. First, the cell viability was analyzed post-mixing with either the mechanical method or the passive mixing unit. Next, the cartilage and skin constructs were cultured and analyzed for relevant histological markers.
Cell distribution appeared homogenous in both cases. However, the action of mixing by hand using a spatula (Figure 2b) has more variance than mixing with a passive mixing unit (Figure 2a). The extent, speed, and mixing technique with a spatula is highly dependent on the user. However, blending cells into a bioink with a passive mixing unit standardizes mixing and minimizes variation across batches. Furthermore, the 30, 60, and 90 s mixing time may not be sufficient for the mixing of a large quantity of cells into a large quantity of bioink. More rigorous blending through mixing with a spatula may be necessary. In comparison, utilizing a passive mixing unit better maintains the mixing ratio of the cells to bioink during blending and ensures that a homogeneous mixing is performed. Additionally, sample loss is a concern with traditional mixing techniques where bioink can be left behind in the Petri dishes, tubes, and mixing tools due to their high viscosity. The cell viability was high for mixing with a mixing unit (> 90%) 1, 2, or 3 times. When the passive mixing unit was utilized, blending took about 1 min at a slow steady blending rate. While mixing with a spatula exhibited high viability after mixing for 30 and 60 s, greater than 90 s of mixing resulted in a significant decrease in viability compared to the other groups (77.9 ± 14%, p <0.05) (Figure 2c). This may be due to excessive mixing that causes damage to the cells. Long-term live/dead staining after 14 days and 28 days of culture is shown in Supplementary Figure 1. Cells begin spreading by day 14, and are highly spread by day 28, and indicate good viability.
After culture, both cartilage and skin tissue analogs were analyzed through histology. Analysis of cartilage constructs at days 0, 14, and 28 shows an increase in the quantity and coverage of GAGs as demonstrated through an Alcian Blue stain (Figure 3a-3c). The absence of the stain is noticed at day 0, while at day 14 staining is limited to locations proximal to the cells. However, by day 28 of culture, the Alcian blue is found throughout the construct, demonstrating the formation of a chondrocytic ECM. Bioprinted skin constructs were analyzed through histological staining of collagen I utilizing a Masson's Trichrome stain (Figure 3d-3f). Similar to the cartilage constructs, day 0 samples exhibited no collagen deposition. By day 14 of culture, substantial deposits of collagen were noted around the cells and along the surface of the construct. This was further enhanced by day 28, as dense collagen layers were found on the construct surface and within the bulk.
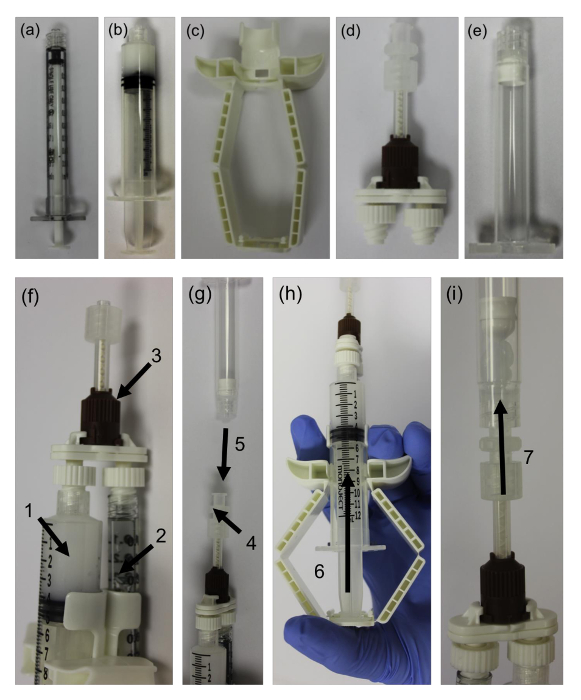
Figure 1: Passive Mixing Unit System. (a) 1 mL syringe for cell suspension, (b) 12 mL syringe with bioink, (c) dispensing unit, (d) passive mixing unit, (e) filling cartridge, (f) assembly of the bioink syringe (1) and cell suspension syringe (2) in the dispensing unit, attachment of the passive mixing unit to the end of both syringes (3), (g) attachment of the female-female Luer lock connector (4) and attachment of the filling cartridge (5) completes the assembly, (h) compress the dispensing unit to prime the mixing unit (6), (i) continue to push down on the dispensing unit to mix the cell suspension with the bioink and dispense into the filling cartridge (7). Please click here to view a larger version of this figure.
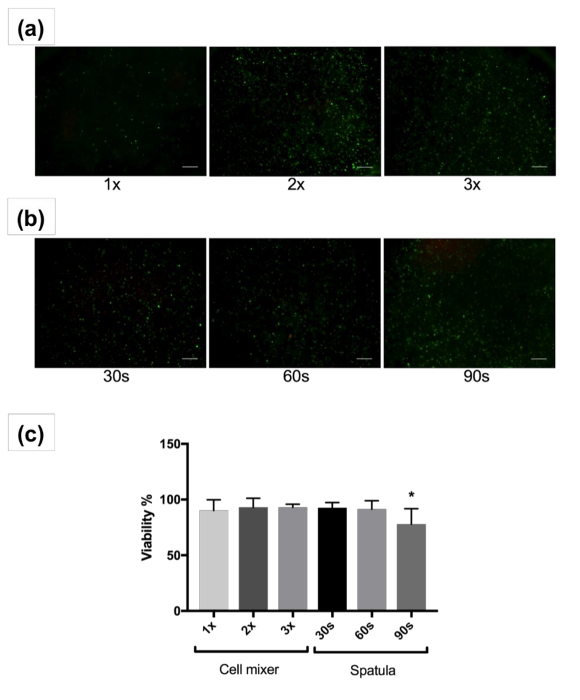
Figure 2: Cell Viability Images and Analysis. Representative images showing live (green) and dead (red) human fibroblasts after mixing with the passive mixing unit 1, 2, or 3 times (a) or spatula for 30, 60, or 90 seconds (b) and 3D culture for 1 day. Images at 4X magnification are shown. Scale bars indicate 200 µm. (c) Percent average viability of human fibroblasts after mixing with the passive mixing unit or spatula and 3D culture for 1 day. Error bars show standard deviation of mean, * 0.005. Please click here to view a larger version of this figure.
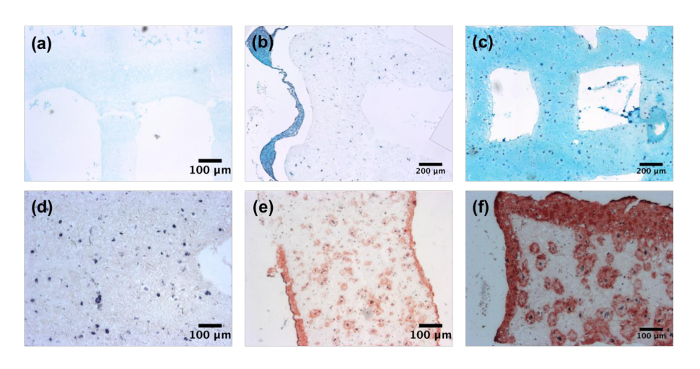
Figure 3: Tissue Specific Extracellular Matrix Deposition. Bioprinted cartilage constructs stained for glycosaminoglycans utilizing Alcian blue at (a) day 0, (b) 14, and (c) 28. Images captured at 5X magnification. Bioprinted skin constructs stained for collagen I using Masson's Trichrome (d) day 0, (e) 14, and (f) 28. Images captured at 5X magnification. Please click here to view a larger version of this figure.
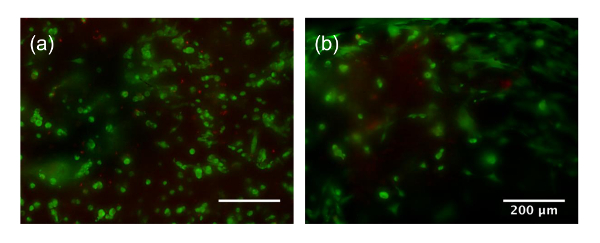
Supplementary Figure 1: Cell Viability at day 14 (a), and day 28 (b). Scale bar is 200 µm, green refers to live cells, red refers to dead cells. Please click here to view a larger version of this figure.
Discussion
As demonstrated in this manuscript, pre-cellularized bioinks were bioprinted to fabricate either cartilage or skin analogs that were cultured for up to 4 weeks. Cell viability after blending using the passive mixing unit technique was higher than the traditional mixing methods. Additionally, the simplification of the blending process using the mixing unit minimizes the number of handling steps and allows for better consistency in the extent of mixing. Ultimately, this results in better reproducibility in both cell distribution within the bioink, and across constructs and experimental groups. Deposition of tissue specific ECM components such as GAGs and collagen I was observed for both the cartilage and skin analogs, respectively after 4 weeks of culture. This indicates the flexibility of both the mixing technique and the chosen construct geometry to fabricate different tissue targets. The fabrication of both cartilage and skin analogs exhibited the flexibility of both the use of a nanocellulose-alginate bioink and the bioprinting technique for distinct engineered tissue targets. We will discuss two components of the study below: (1) the passive mixing unit technique, and (2) the behavior of cells within cartilage and skin analogs.
The development and the optimization of a technique for the blending of a cell suspension into a bioink was paramount for the fabrication of these constructs. Unlike traditional low viscosity hydrogel materials, the high viscosity of the bioinks resulted in difficulty in pre-cellularization of a bioink prior to printing. As an alternative to the traditional method of cell incorporation into a bioink, a protocol for the one-step mixing of a cell suspension into a viscous bioink was developed and discussed. Additionally, the practical application of this mixing technique in the fabrication of tissue constructs was evaluated. This one-step mixing approach has several advantages over traditional mixing techniques included controlled mixing ratio, minimization of high and irregular shear stresses, and a closed system to eliminate sample close in mixing vessels. However, there are several critical steps in this technique to ensure its advantages over traditional methods for cell/bioink blending. It is critical to ensure that the cells in the cell syringe are suspended and well mixed prior to blending. Too large of a time gap between the lifting and transfer of the cells in the syringe and starting the mixing process can cause the cells to sediment, which will result in uneven mixing and distribution into a printed filament. If sedimentation is observed, simple inversion (2 – 3 times) of the passive mixing unit assembly immediately prior to mixing is sufficient to resuspend the cells and ensure a uniform distribution prior to blending. It is also critical that air bubbles in the syringes are eliminated or minimized prior to ensure that the mixing ratio remains constant. If large air bubbles remain in the bioink cartridge prior to blending, it is recommended that the solution be run through the mixing unit an additional time to ensure thorough mixing. If air bubbles remain, gentle tapping of the printing cartridge/syringe can displace residual air bubbles. Additionally, if multiple material blending (several mixing units or materials) is necessary for more complex constructs, the concentrations of the bioinks/cell suspensions to be mixed must be adjusted to ensure that after diluting through blending, the proper final concentration is still achieved.
In comparison to other blending techniques, the passive mixing unit is a novel approach to cellularize bioinks. Unlike other mixing techniques such as dual syringe mixing, or stirring with a spatula or other tool, the passive mixing unit allows more consistency in blending across batches and users. The nature of manual mixing techniques, such as a spatula mixing, results in greater user-to-user variation in the extent of and rate of mixing. Additionally, closed systems such as the passive mixing unit and dual syringe mixing have little to no sample loss compared to manual mixing within a Petri dish or tube.
Both tissue analogs fabricated in this manuscript consisted of a single type of bioink and either one or two cell types. However, the bioprinting of tissue analogs that consist of architectures that contain regions of distinct bioinks with distinct cell types may be necessary for the fabrication of more physiological tissue constructs20,21,22. More specialized bioinks with unique compositions or functionalities may be generated through the blending of several types of existing bioinks together to create multimaterial bioinks that have distinct compositions or functionalities23,24. This may be particularly important at tissue transition regions such as the skin to subcutaneous layer or the cartilage to bone region of a tissue where a gradient of bioink composition25,26 may be necessary to ensure development of these critical regions found in native tissues27. Additionally, a mixing unit could be utilized to blend in growth factors and morphogens into the bioinks prior to bioprinting. The elimination of sample loss with the closed mixing system is attractive for the use of expensive and low concentration bioactive factors, where sample loss can result in a change to their final concentration within the bioprinted construct, particularly when gradients are involved.
A limitation with any mixing technique, including the passive mixing unit utilized in this study, is the risk of damage to mechanically sensitive cell types. For example, isolated stem cells such those from the bone marrow or embryo, or induced pluripotent stem cells are more susceptible to mechanical damage28,29. The act of mixing imparts abnormal mechanical stresses on the cells due to the shear forces involved in the mixing process, and they must be balanced to preserve viability30. While the use of primary fibroblasts and chondrocytes in this study resulted in good viability (Figure 2), more studies are necessary to determine safe mixing rates and ratios for more sensitive cell types. Until then, it is recommended that the mixing be performed under a steady slow rate to maximize viability. Additionally, the cell density chosen in this study was determined based on previous studies1. This cell density may not be ideal for all cell types. In particular, blending cells at a higher concentration may result in increased cell-cell contacts that may improve cell viability and tissue formation31,32. In a similar vein, the passive mixing unit could be applicable to blending cell spheroids or other cell aggregates33 into bioinks.
As proposed and demonstrated in this study, a passive mixing unit system can rapidly blend a cell suspension into a viscous bioink. While the risk for mechanical damage to the cells still remains, the approach allows more consistency in blending within a single study and across users. Pre-cellularized bioinks utilizing this approach were used to fabricate both cartilage and skin analogs that were cultured for up to 4 weeks, and demonstrated deposition of tissue specific ECM markers. Future studies will focus on the utilization of the passive mixing unit system to blend specialized bioinks from standardized bioinks for the fabrication of more complex tissue analogs.
開示
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| STARTINK Kit | CELLINK | SK0001 | Kit that contained all the elements for the CELLMIXER procedure |
| Live/Dead Kit | Life Technologies | L3224 | Kit for the analysis of cell viability after mixing |
| Masson's Trichrome | Sigma Aldrich | HT15-1KT | Kit for the analysis of collagen I deposition in the skin constructs |
| Alcian Blue | Sigma Aldrich | B8438-250ML | Kit for the analysis of glycosaminoglycan deposition in the skin constructs |
| INKREDIBLE+ bioprinter | CELLINK | Gen1+ | Printer utilized in the study |
| DMEM with Glutamax | Thermofisher | 10566016 | Media for culture of the cells |
| 10% fetal bovine serum | Thermofisher | A3160402 | Media supplement |
| Penicillin-Streptomycin | Life Technologies | 15070063 | Media supplement |
| live cell imaging solution | thermofisher | A14291DJ | component utilized using live/dead imaging |
| inverted microscope | Olympus | IX73 | microscope utilized |
| a digital color camera | Olympus | XC10 | microscope camera utilized |
| cellSens imaging software | Olympus | n/a | stock software with the microscope |
| ImageJ | NIH | n/a | open source image analysis software |
| GraphPad Prism 7 | GraphPad | n/a | software for statistical analysis |
| Slic3r software (v1.2.9) | Slic3r | n/a | open-source software to convert .stl file to gcode |
| primary adult human dermal fibroblasts | ATCC | PCS-201-012 | cell source for fibroblasts |
参考文献
- Avila, H. M., Schwarz, S., Rotter, N., Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting. 1, 22-35 (2016).
- Lee, V., et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 20 (6), 473-484 (2013).
- Costantini, M., et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials. 131, 98-110 (2017).
- Fedorovich, N. E., et al. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng Part C Methods. 18 (1), 33-44 (2011).
- Vijayavenkataraman, S., Lu, W. F., Fuh, J. Y. H. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication. 8 (3), 032001 (2016).
- Peltola, S. M., Melchels, F. P. W., Grijpma, D. W., Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 40 (4), 268-280 (2008).
- Xu, T., Jin, J., Gregory, C., Hickman, J. J., Boland, T. Inkjet printing of viable mammalian cells. Biomaterials. 26 (1), 93-99 (2005).
- Cui, X., Dean, D., Ruggeri, Z. M., Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 106 (6), 963-969 (2010).
- Parsa, S., Gupta, M., Loizeau, F., Cheung, K. C. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication. 2 (2), 025003 (2010).
- Derby, B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res. 40, 395-414 (2010).
- Colosi, C., et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 28 (4), 677-684 (2016).
- Ober, T. J., Foresti, D., Lewis, J. A. Active mixing of complex fluids at the microscale. Proc Natl Acad Sci. 112 (40), 12293-12298 (2015).
- Shabnam, P., Madhuja, G., Frédéric, L., Karen, C. C. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication. 2 (2), 025003 (2010).
- Chahal, D., Ahmadi, A., Cheung, K. C. Improving piezoelectric cell printing accuracy and reliability through neutral buoyancy of suspensions. Biotechnol Bioeng. 109 (11), 2932-2940 (2012).
- Niu, X., Lee, Y. -. K. Efficient spatial-temporal chaotic mixing in microchannels. J Micromech Microeng. 13 (3), 454 (2003).
- Liu, R. H., et al. Passive mixing in a three-dimensional serpentine microchannel. J Microelectromech Syst. 9 (2), 190-197 (2000).
- Strober, W. Trypan blue exclusion test of cell viability. Curr protoc immunol. , (2001).
- Scott, J., Dorling, J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochem Cell Biol. 5 (3), 221-233 (1965).
- Garvey, W. Modified elastic tissue-Masson trichrome stain. Stain technol. 59 (4), 213-216 (1984).
- Xu, T., Zhao, W., Zhu, J. -. M., Albanna, M. Z., Yoo, J. J., Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 34 (1), 130-139 (2013).
- Kolesky, D. B., et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 26 (19), 3124-3130 (2014).
- Kolesky, D. B., Homan, K. A., Skylar-Scott, M. A., Lewis, J. A. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci. 113 (12), 3179-3184 (2016).
- Rutz, A. L., Hyland, K. E., Jakus, A. E., Burghardt, W. R., Shah, R. N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater. 27 (9), 1607-1614 (2015).
- KÖpf, M., Campos, D. F. D., Blaeser, A., Sen, K. S., Fischer, H. A tailored three-dimensionally printable agarose-collagen blend allows encapsulation, spreading, and attachment of human umbilical artery smooth muscle cells. Biofabrication. 8 (2), 025011 (2016).
- Gurkan, U. A., et al. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Mol Pharm. 11 (7), 2151-2159 (2014).
- Ma, Y., Ji, Y., Huang, G., Ling, K., Zhang, X., Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication. 7 (4), 044105 (2015).
- Lu, H. H., Thomopoulos, S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 15, 201-226 (2013).
- Aguado, B. A., Mulyasasmita, W., Su, J., Lampe, K. J., Heilshorn, S. C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 18 (7-8), 806-815 (2011).
- Parisi-Amon, A., Mulyasasmita, W., Chung, C., Heilshorn, S. C. Protein-Engineered Injectable Hydrogel to Improve Retention of Transplanted Adipose-Derived Stem Cells. Adv Healthc Mater. 2 (3), 428-432 (2013).
- Qiu, X., De Jesus, J., Pennell, M., Troiani, M., Haun, J. B. Microfluidic device for mechanical dissociation of cancer cell aggregates into single cells. Lab Chip. 15 (1), 339-350 (2015).
- Cukierman, E., Pankov, R., Yamada, K. M. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 14 (5), 633-640 (2002).
- Loessner, D., et al. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 31 (32), 8494-8506 (2010).
- Lin, R. Z., Chang, H. Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 3 (9-10), 1172-1184 (2008).

