A Microfluidic Flow Chamber Model for Platelet Transfusion and Hemostasis Measures Platelet Deposition and Fibrin Formation in Real-time
概要
This paper describes an experimental model of hemostasis that simultaneously measures platelet function and coagulation. Platelet and fibrin fluorescence is measured in real-time, and platelet adhesion rate, coagulation rate, and onset of coagulation are determined. The model is used to determine platelet procoagulant properties under flow in concentrates for transfusion.
Abstract
Microfluidic models of hemostasis assess platelet function under conditions of hydrodynamic shear, but in the presence of anticoagulants, this analysis is restricted to platelet deposition only. The intricate relationship between Ca2+-dependent coagulation and platelet function requires careful and controlled recalcification of blood prior to analysis. Our setup uses a Y-shaped mixing channel, which supplies concentrated Ca2+/Mg2+ buffer to flowing blood just prior to perfusion, enabling rapid recalcification without sample stasis. A ten-fold difference in flow velocity between both reservoirs minimizes dilution. The recalcified blood is then perfused in a collagen-coated analysis chamber, and differential labeling permits real-time imaging of both platelet and fibrin deposition using fluorescence video microscopy. The system uses only commercially available tools, increasing the chances of standardization. Reconstitution of thrombocytopenic blood with platelets from banked concentrates furthermore models platelet transfusion, proving its use in this research domain. Exemplary data demonstrated that coagulation onset and fibrin deposition were linearly dependent on the platelet concentration, confirming the relationship between primary and secondary hemostasis in our model. In a timeframe of 16 perfusion min, contact activation did not take place, despite recalcification to normal Ca2+ and Mg2+ levels. When coagulation factor XIIa was inhibited by corn trypsin inhibitor, this time frame was even longer, indicating a considerable dynamic range in which the changes in the procoagulant nature of the platelets can be assessed. Co-immobilization of tissue factor with collagen significantly reduced the time to onset of coagulation, but not its rate. The option to study the tissue factor and/or the contact pathway increases the versatility and utility of the assay.
Introduction
Primary and secondary hemostasis were originally conceptualized as two relatively separable biochemical processes. Primary hemostasis was viewed as a major contributor in arterial flow conditions, with a key role for platelets, while secondary hemostasis was seen to dominate coagulation under venous blood flow, allowing the protease cascade to form insoluble fibrin. The last few decades have reshaped this traditional view substantially, acknowledging that platelet activation and coagulation are interdependent and are equally important processes during physiological and pathological hemostasis in all (non-extreme) hydrodynamic milieus. This view has been translated to the clinic, where assays devised to comprehensively measure whole-blood coagulation are increasingly being used, albeit with many remaining questions on relevance, applicability and utility1.
We recently published a functional analysis of platelet concentrates using microfluidic flow chambers coated with fibrillar collagen in an in vitro model of transfusion2, with samples containing normal amounts of red blood cells, plasma, and platelets. This method uses heparin or hirudin anticoagulated blood, implying no or limited involvement of secondary hemostasis, which is dependent on thrombin generation and ionized calcium (Ca2+). To support thrombin formation and thus to include all aspects of hemostasis under microfluidic flow, we developed a complementary method on the same technical platform, now including free Ca2+. In this way, platelet deposition and fibrin formation can be studied, again in a model of platelet transfusion, to assess platelet concentrate quality.
In this method, the platelets and fibrinogen are labeled with fluorophores that have fully separated emission spectra. Dual color, real-time video microscopy then permits the analysis of primary platelet adhesion, as well as coagulation initiation and fibrin deposition. An important variable in this type of assay is recalcification, because the addition of Ca2+ to static blood, prior to perfusion, will inevitably cause contact-initiated coagulation in the container. This initiation will bias the readout due to clogging of tubing and biochip inlets prior to perfusion. Therefore, in our method, citrate anticoagulated blood and a Ca2+-containing buffer are pumped separately through the upper legs of a Y-shaped inlet chamber. The components are mixed during perfusion before entering an analysis chamber coated with collagen alone or in combination with recombinant human tissue factor (rhTF). By adapting the flow velocities of the separate pumps and the concentration of Ca2+ in the buffer, a 10% dilution of the final blood sample takes place.
Using this extended protocol, we demonstrate the contribution of coagulation factor XII (FXII) and platelet concentration to contact pathway coagulation initiation under flow. Our data also show that by coating rhTF alongside collagen, the tissue factor pathway can be measured concomitantly.
Protocol
This protocol follows the institutional ethical guidelines for research on human samples, and informed consent was obtained from all donors involved. Approval for the experiments described here was obtained from the institutional review board of the Antwerp University Hospital (approval number 16/10/120).
NOTE: Temperature indications are always room temperature, unless specified.
1. Microfluidics Setup
- Prepare the microfluidic flow chamber channels, tubing, and connecting pins
- Resuspend the collagen stock vial by vortex mixing, and then dilute in the isotonic glucose solution provided by the manufacturer to a final concentration of 50 µg/mL.
NOTE: Use equine tendon collagen, mainly made up of type I fibrils. The equine collagen type I is often referred to as "Horm" collagen and is the gold standard for this type of assay, for both historical and biological reasons. Human type III collagen can also be used, but these fibrils coat less well, and the platelet response is not as strong. Other coating surfaces can also be used, such as von Willebrand factor, fibrinogen, fibronectin, laminin, vitronectin, thrombospondin-1, or combinations of these3. - Take a new, disposable microfluidic biochip with eight straight, parallel channels and dimensions, in mm, of 0.4 W x 0.1 H x 20 L. Pipet 0.8 µL of collagen into the channel(s) of the biochip on one end and label this as the outlet. Fill just 5/6 of the channel length so that the collagen fibrils are not coated at the channel inlet, because this can cause clogging, which obstructs an efficient perfusion.
- Incubate the biochip at 4 °C for at least 4 h in a humidified and sealed container.
- To study tissue factor (extrinsic)-mediated coagulation in combination with the contact pathway (intrinsic), coat the channels with collagen and recombinant human tissue factor (rhTF).
- Dilute rhTF in 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered saline (HBS; 0.9% (w/v) NaCl, pH 7.4, 1:3,000, stock concentration: 4.5 nM).
- Remove the collagen-coating solution via the outlet. Pipet 0.8 µL of rhTF in HBS (dilution 1/500 of stock concentration, final concentration: 9 pM) into the outlet of the channel, filling 5/6 of the channel length, similar to the collagen-coating strategy.
- Incubate the biochip for an additional 30 min in a humidified and sealed container.
- Fill the coated channels with blocking buffer (1.0% (w/v) bovine serum albumin and 0.1% (w/v) glucose in HBS), starting from the other end (labeled as the inlet). Fill an equal number of Y-shaped channels with the same blocking buffer in a mixing biochip.
- Make sure all channels and channel arms are completely filled with blocking buffer. Incubate both biochips for at least 1 h in a humidified and sealed container.
- For each channel in use, prepare two tubing of 8 cm long, one 2 cm long, and one 46 cm long. Place a pin in one end of the three smaller tubing and in both ends of the longest tubing.
- Resuspend the collagen stock vial by vortex mixing, and then dilute in the isotonic glucose solution provided by the manufacturer to a final concentration of 50 µg/mL.
- Prepare the rinsing pump
- Rinse all pump fluidics and the connected tubing with distilled water.
- Degrease the biochip's casting with a precision dust-free wipe and denatured alcohol to remove prints and dust.
- Fill all tubing with blocking buffer before connecting them to the biochips.
- Fix the coated biochip on the automated microscope stage. Fix the mixing biochip at the same height as the microscope stage using a laboratory scissor jack.
- Connect the coated biochip with the mixing biochip using the longest tubing, 46 cm long. Make sure both biochips are at a distance so that the tubing forms a flexible but straight line.
- Attach a syringe connector pin into the Luer-compatible tubing of the rinsing pump. Connect it to the free end of the 2-cm tubing and fix the other end to the outlet of the coated biochip.
- Fix the two 8-cm tubing to the mixing biochip inlet using its pins. Put the free end of each tubing in a vial of HBS. Rinse all tubing and all channels with 1 mL HBS.
NOTE: HBS flows from the vial through the 8-cm tubing to the mixing chip and through the 46-cm tubing to the coated chip (flowing from the uncoated to the coated part) due to the pulling force of the rinsing pump. This step removes the blocking buffer and poorly adhered collagen (or rhTF in double-coated biochips).
- Prepare the perfusion pumps
- Open the driver software of the perfusion pumps. Initialize the pumps by double-clicking on the syringes icon in the software.
- Select the type of syringe that will be used: 1 mL syringes for the coagulation buffer and 2 mL syringes for the reconstituted blood.
- Connect the syringes to the biochip construction
- Disconnect the rinsing pump and all tubing, except for the one connecting the two biochips. The disconnected tubing is reused in the following steps.
- Connect the 2-cm tubing with its syringe connector pin to the Luer lock of a thick-walled waste tubing. Fill the thick-walled tubing with standard pepsin solution using a syringe.
NOTE: The addition of pepsin to the waste tubing inhibits and lyses post-perfusion clotting and therefore prevents clogging of the system at the back end. - Secure the open end of the thick-walled tubing with a Kocher clamp. Place a suitable waste container with bleach underneath to collect the flow-through.
- Fix the tubing with its pin to the outlet of the coated biochip.
2. Preparation of Blood Samples
- Collect blood from a healthy volunteer and separate its components4
- Collect blood in an evacuated ethylenediamine-tetraacetic acid (EDTA) container. Use this sample only for performing a complete blood count (CBC) using an automated hematology analyzer.
- Collect blood in evacuated sodium citrate containers. 5 mL of blood is required per channel.
- Place the sample(s) on a horizontal rotator pending blood reconstitution.
NOTE: The assay should be completed within 3 h of phlebotomy. Platelet concentrate and whole-blood samples are ABO/RhD blood group matched. - Centrifuge for 13 min at 250 x g to prepare platelet-rich plasma (PRP). Do not use the centrifuge brake to prevent disturbance of the loosely packed pellet.
- Transfer the PRP to a fresh conical centrifugation tube. Centrifuge for 10 min at 4,500 x g (with brake) to pellet the platelets and prepare platelet-poor plasma (PPP). Discard the platelet pellet. Incubate the PPP in a water bath at 37 °C.
- Transfer the packed red blood cells to a fresh conical tube by perforating the bottom of the original one with a 21 G needle.
NOTE: The residual platelet count in the packed red cell fraction is 11 ±4 x 103 per µL (mean ± SD, n = 10).
- Reconstitute blood
- Determine the hematocrit of the packed red blood cells prepared in step 2.1.6 using an automated hematology analyzer.
- Determine the platelet concentration of the platelet concentrate that will be used for analysis using an automated hematology analyzer.
- Calculate the volume of packed red blood cells, platelet concentrate, and PPP that will yield a 40% hematocrit and 250 x 103 platelets/µL in a 2.5 mL final volume. Mix these to prepare reconstituted blood and perform a CBC.
NOTE: Other target titers of cells can be used, depending on the research question. - Prepare a "blank" control sample in which the volume of platelet concentrate is replaced by the same volume of saline to determine the concentration of "endogenous" platelets (i.e., non-blood bank platelets).
- Label the reconstituted blood for platelets and fibrinogen
- Pipet 13 µL of a 10.7 mg/mL solution of Alexa Fluor 405-labeled fibrinogen (70 µg/mL final concentration) in a test tube and add 1 mL of reconstituted blood.
NOTE: Fibrinogen was labeled using a commercially available kit according to the manufacturer's manual. - Mix another 1 mL of reconstituted blood in a test tube containing 2 µL of 1 mM DiOC6 (1 µM final concentration) or containing 2 µL of 5 mM Calcein AM (5 µM final concentration). Add this to the tube containing the blood with fibrinogen, giving a final volume of 2 mL of platelet- and fibrinogen-stained blood.
NOTE: The choice of dye can depend on the research question or the preference of the researcher5. Be aware that excitation of any dye can cause photochemical artifacts. - Gently mix by inverting. Incubate the sample for 10 min at 37 °C.
- Pipet 13 µL of a 10.7 mg/mL solution of Alexa Fluor 405-labeled fibrinogen (70 µg/mL final concentration) in a test tube and add 1 mL of reconstituted blood.
- Prepare the coagulation buffer
- Prepare coagulation buffer containing 10 mM CaCl2 and 3.75 mM MgCl2 in HBS. Prepare 1 mL of coagulation buffer for one channel.
- Filter-sterilize the coagulation buffer through a 0.2 µm filter. Pre-warm this to 37 °C.
3. Perfusion Assay
- Focus the microscope optics to the collagen fibers adhered to the bottom of the channel. Use phase contrast or differential interference contrast (DIC). Select "Set current Z for selected tile regions" in the experiment software to digitally fix the selected focus.
- Define a region of interest (ROI) in the selected channel using the acquisition software of the microscope.
NOTE: The ROI can be any surface area arbitrarily chosen within a perfusion channel. It is advised not to analyze thrombus formation and fibrin generation close to the inlet and outlet in order to avoid acquiring images of unevenly distributed thrombi. The ROI surface area should contain a significant number of thrombi to allow for the leveling out of signal variability by individual platelets. The location of the ROI in the xy-plane of the channel is always fixed and determined prior to perfusion. If required, the uncoated part of the channel can be included in the analysis to determine if platelets bind nonspecifically to the biochip plastic. - Prepare samples for perfusion
- Mount a 1 mL syringe containing 1 mL of pre-warmed coagulation buffer in the perfusion pump.
- Mix the pre-warmed reconstituted blood by gently inverting and load 2 mL in a 2 mL syringe.
- Make sure all air is removed from both syringes and connect each to an 8-cm tubing using a syringe connector pin.
- Prime the connectors and tubing of both syringes at high speed (400 µL/min) and stop when everything is filled with coagulation buffer or blood.
NOTE: By priming the tubing, the coagulation buffer and reconstituted blood will be immediately mixed in the Y-shaped channel of the mixing biochip when starting the experiment, avoiding the introduction of air to the perfusion chambers.
- Using pins, fix the other end of both tubing to the split legs of the Y-shaped channel in the mixing biochip. The bottom leg is used for perfusing coagulation buffer and the upper leg for reconstituted blood.
- Start the experiment by initiating perfusion at 4.4 µL/min for coagulation buffer and 44 µL/min for reconstituted blood. Remove the Kocher clamp at the outlet of the waste tubing to permit perfusion.
NOTE: Flow rate can be changed depending on the research question. Start a stopwatch to determine the time between the initiation of the experiment and the start of real-time image acquisition. - Record images every 10 s for 30 min in real time using the acquisition and experiment software of the microscope. Start recording when the mix of reconstituted blood and coagulation buffer enters the coated biochip mounted on the microscope stage.
NOTE: Other time series can be used depending on the experimental setup. Use a 100X magnification (10X objective and 10X lenses).
4. Terminating Experiment
- Terminate the pumps and image acquisition after 30 min or earlier, if the signal is saturated. Detach all tubing and syringes and discard them as biohazardous waste.
5. Data Analysis
- Determine thrombus growth (green fluorescence) and fibrin formation (violet fluorescence) kinetics with the image analysis software. The following commands are specific for the software used here:
- Open the plugin Processing: stitching to stitch the side-by-side images per time point.
- Open the plugin Image Analysis to determine the surface coverage of the platelets.
- Open the plugin Measure. Define the ROI by drawing a rectangle region including all thrombi and fibrin fibers; in this protocol, the ROI is a rectangle measuring 637 mm2.
Select the tab All Views to include all recorded time points. - Use Create Tables to automatically generate a spreadsheet that will contain the mean fluorescence intensity value of the selected rectangle region of each time point.
- Save the spreadsheets in xml format.
- Open a spreadsheet in a spreadsheet program for further calculations.
- Subtract the initial (background) value of all data.
- Plot the fluorescent signal as a function of the perfusion time for both the green (platelets) and the violet (fibrin) dyes.
NOTE: Take into account the time between pump initialization and the actual starting point of image acquisition. Thrombus formation is generally a two-step process in this model: (1) "slow" platelet adhesion (i.e., adhesion) to immobilized collagen followed by (2) coagulation-driven thrombus growth with a "rapid" increase in both platelet binding (i.e., accumulation) and fibrin deposition (i.e., coagulation) (Figure 1). - Calculate the slope of platelet adhesion and accumulation by linear regression; this slope yields platelet thrombus growth kinetics in each phase of its formation.
- Calculate the slope of fibrin coagulation and extrapolate this line to intercept the X-axis, determining the moment of onset.
Representative Results
The analysis of real-time raw data is described in Figure 1. First, platelets adhere to the reactive surface, resulting in a steady increase in recorded green fluorescence (Figure 1A, i), called adhesion. During this phase, there is little violet fluorescence, indicating that fibrin is not or is only marginally formed (Figure 1B). Upon initiation of coagulation, violet-fluorescing fibrin deposits rapidly (iii), and during that time, platelet green fluorescence increases at about the same rate, designated here as platelet accumulation (ii). A single experiment thus returns three rates of fluorescence increase (i, ii, and iii) as a surrogate marker for the velocity at which platelet and fibrin deposition takes place in this model. Furthermore, a moment of coagulation onset (moment-of-onset (iv)) is extrapolated, which is a determinant of platelet procoagulant potential.
In the absence of TF, coagulation initiation is slow and primarily runs through the contact pathway where the intrinsic tenase complex activates FX via direct activation of FIX6 by FXI and/or FXII. To demonstrate FXII dependence, 4 µM of corn trypsin inhibitor (CTI) was added to inhibit activated FXII (FXIIa)7. This inhibition did not affect platelet adhesion (Figure 2A), but coagulation did not start for the arbitrarily defined total duration of the perfusion experiment (Figure 2B and Supplementary Video 1).
In vitro, the contact pathway can be initiated by foreign materials like glass, or in clinical assays using a mineral material like kaolin. In vivo, activated platelets provide the negative charge8 through the membrane exposure of acidic phospholipids9, like phosphatidylserine, and/or through the release of polyphosphates (polyP)10,11. Our microfluidic real-time assay mimics the latter because, in a range of platelet concentrations, the hemostatic reaction depended on the platelet count (Figure 3). By increasing the number of platelets in the reconstituted sample, the rate of adhesion (Figure 3A), accumulation (Figure 3B, green), and coagulation (Figure 3B, violet) increased linearly. The moment-of-onset significantly shortened (Figure 3C) by increasing platelet concentration, suggesting that a threshold number of (activated) deposited platelets is required to trigger coagulation.
Upon tissue damage in vivo, however, TF-bearing cells will initiate the clotting of blood via the FVIIa-TF extrinsic tenase complex in the presence of Ca2+. This is mimicked in our experimental setup by post-coating the collagen-containing perfusion chambers with lipidated rhTF. In a paired analysis of channels coated with collagen only or in combination with rhTF, coagulation onset was significantly faster (Figure 4A). The rate of platelet adhesion was not linear, so no linear regression could be performed (data not shown). Both the rate of coagulation and platelet accumulation were not different between conditions (Figure 4B-4C, Supplementary Video 2).
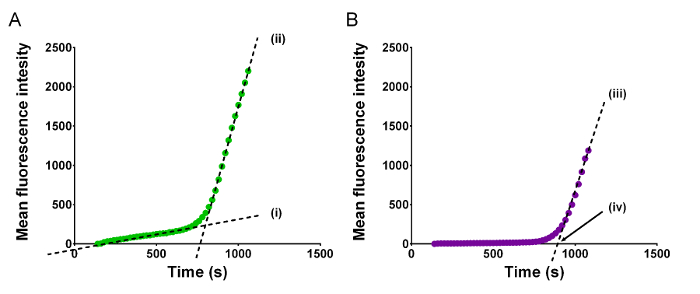
Figure 1: Regression analysis of platelet adhesion and coagulation in microfluidic perfusion chambers. This figure clarifies the parameters derived from real-time fluorescence raw data acquired during recalcified blood flow over a reactive surface. (A) Mean intensity of green fluorescence shows platelet deposition in function of perfusion time. The curve describes a bimodal process beginning with (i) slowly increasing linear platelet adhesion followed by (ii) rapidly growing linear accumulation. Both linear parts of the curve are regressed and the slope of these describes the two rates of thrombus formation by platelet fluorescence; (i) adhesion and (ii) accumulation. (B) Mean intensity of violet fluorescence shows deposition of fibrin in function of time. During platelet adhesion, violet fluorescence is essentially absent, while it quickly develops following initiation of coagulation. The (iv) moment-of-onset is defined as the intercept with the x-axis of the extrapolated linear regression of (iii) the second phase of thrombus formation by fibrin fluorescence designated here as coagulation. Please click here to view a larger version of this figure.
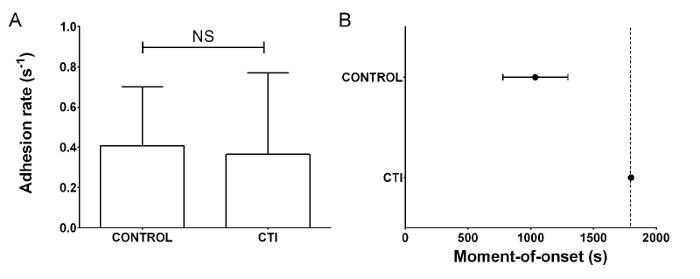
Figure 2: In the absence of TF, coagulation in collagen-coated perfusion flow chambers depends on FXIIa. Microfluidic perfusion of reconstituted blood containing CTI or control buffer (CONTROL) was performed at a shear rate of 1,000 s-1 for a total of 30 min. (A) The rate of platelet adhesion (s-1) was not dependent on CTI. (B) The moment-of-onset for CTI-treated samples was beyond the 30 min limit of the experiment (shown as a dotted line), demonstrating the dependence on FXIIa of this parameter. Bars and dots are mean values, whiskers are standard deviation. Statistical analysis was by paired t-test. (NS = not significant, n ≥3). Please click here to view a larger version of this figure.
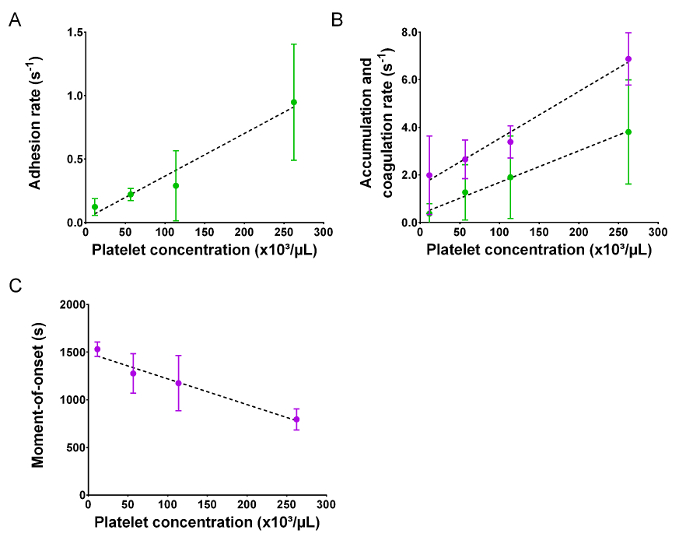
Figure 3: Coagulation under flow depends on the platelet number. Microfluidic perfusion experiments were performed in collagen-coated chambers with reconstituted blood in the presence of Ca2+ and varying platelet concentrations (n ≥3). (A) Rate of platelet adhesion (B) rate of platelet accumulation (green) and coagulation (violet), and (C) moment-of-onset are shown as functions of platelet concentrations in reconstituted blood. Dots are mean values, whiskers are standard deviations. Please click here to view a larger version of this figure.
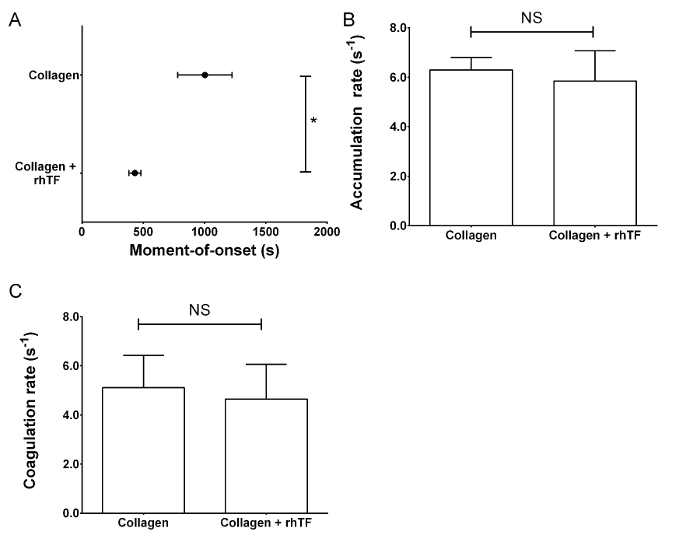
Figure 4: Activation of both TF and contact pathway coagulation significantly shortens the moment-of-onset of coagulation. Reconstituted blood, in the presence of Ca2+, was perfused through chambers coated with collagen alone or with collagen and rhTF to study the contact pathway alone or a combination of the contact and TF pathways. (A) The moment-of-onset of coagulation is significantly shortened in TF-containing flow chambers. (B) The rate of accumulation of platelets during coagulation and (C) the rate of coagulation are not significantly different between collagen only or collagen- and rhTF-coated flow chambers. Bars and dots are mean values; whiskers are standard deviations. Statistical analysis was by paired t-test. (NS = not significant, *P <0.05, n ≥3). Please click here to view a larger version of this figure.
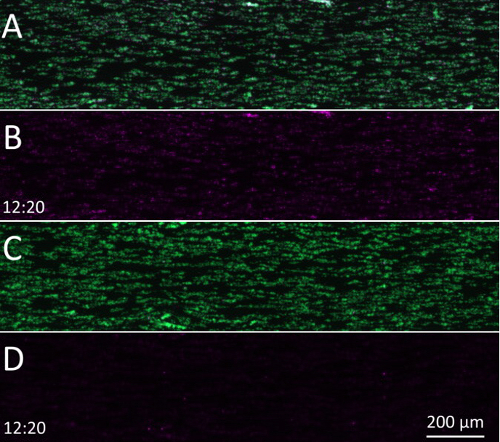
Supplementary Video 1: In the absence of TF, coagulation in collagen-coated perfusion flow chambers depends on FXIIa. The role of the contact pathway is determined in collagen-coated perfusion flow chambers. (A) Overlaid fluorescence image sequence of platelet (green) and fibrin(ogen) (violet) deposition in the absence of CTI. (B) Same sequence as panel A but with the green channel switched off. (C) Overlaid fluorescence image sequence of platelet (green) and fibrin(ogen) (violet) deposition in the presence of CTI. (D) Same sequence as panel C but with the green channel switched off. Please click here to download this video.
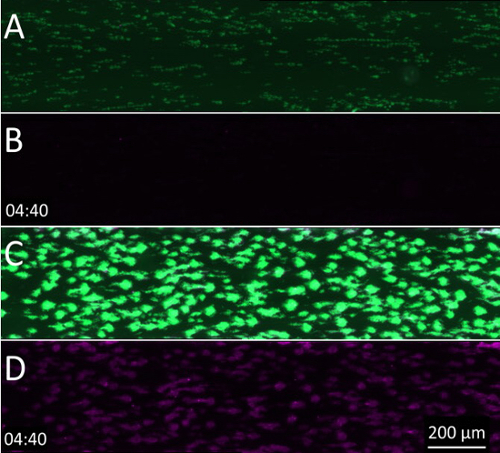
Supplementary Video 2: Activation of both TF and the contact pathway significantly shortens the coagulation moment-of-onset. To study the role of TF, flow chambers coated with collagen alone or with collagen and rhTF were used. (A) Overlaid fluorescence image sequence of platelet (green) and fibrin(ogen) (violet) deposition in flow chambers coated only with collagen. (B) Same sequence as panel A but with the green channel switched off. (C) Overlaid fluorescence image sequence of platelet (green) and fibrin(ogen) (violet) deposition in flow chambers coated with both collagen and rhTF. (D) Same sequence as panel C but with the green channel switched off. Please click here to download this video.
Discussion
The transfusion of platelet concentrates is prescribed for the thrombocytopenic patient to prevent or stop bleeding. Its clinical impact was recently highlighted in a historical review of acute leukemia12, recalling that increased survival rates of pediatric patients in the sixties and seventies were largely attributable to improvements in (platelet) transfusion medicine. Platelet concentrates are also used to stem bleeding in acute trauma or during surgery. In these conditions, platelets with excellent procoagulant properties that rapidly initiate hemostasis are preferred, but platelet concentrates are prepared from donations of voluntary donors and, unlike pharmacological medication, are standardized by preparation only, not by (chemical) composition. Therefore, several questions in the field of blood banking are unanswered, including those on optimal donation modalities, storage conditions, and transfusion practices. In the field of platelet (transfusion) biology, many questions on cell clearance from circulation, the contribution of transfused platelets to hemostasis, or their immunology also remain unanswered.
Numerous laboratory techniques for platelet function analysis are available, but these mostly address a singular aspect of platelet function, like aggregation or degranulation13. To broadly assess platelets and platelet concentrates, comprehensive models of hemostasis are indispensable, and these must include hydrodynamics. Blood rheology is crucial to correctly interpret platelet behavior during hemostasis14,15 or thrombosis16, even if anticoagulation prevents Ca2+ and/or thrombin to participate in the presence of citrate or heparin, respectively2. To study the interplay between coagulation and platelet function under flow17,18, normal thrombin generation is required, and therefore, free Ca2+ as well. The experimental setup for studying hemostasis under these conditions is complex, because measures to prevent "artifact" or uncontrolled activation of coagulation should be taken as much as possible. Furthermore, many specialized research groups use custom-made hardware19,20, which causes substantial inter-laboratory variability5. Typical limitations of this approach are the use of rectangular vessels, non-pulsatile flow profiles, and non-human surface coatings5. The assay we describe here uses commercially available tools and may therefore be suited for standardization.
Because the coagulation cascade reaction is easily activated once blood is not contained within the human body, controlled recalcification is a pivotal step. To achieve this, addition of Ca2+ needs to be postponed to just before perfusion over the reactive collagen surface, because when the Ca2+ buffer is supplied in bulk to static blood, coagulation inevitably takes place, eventually clogging the tubing and biasing data interpretation (data not shown). The solution to this problem is to pump the Ca2+ buffer and the blood separately, allowing for mixing by convective and diffusive forces during perfusion on its way to the analysis chamber. Complete mixing is achieved in a 46-cm segment of tubing between the mixing and analysis chambers. This length was calculated for the optimal dwell time of reagents to mix based on fundamental laws of mass and convective transport21 during perfusion at the flow rate used (1,000 s-1). This approach proved controllable and reproducible.
Contact activation of coagulation is sometimes viewed as an artifact of simple blood sampling and to be avoided when interpreting clotting times. Therefore, we demonstrated that, in the absence of inhibitors, contact-induced coagulation starts at 16.7 min (±3.7 min) of perfusion. In the presence of CTI to inhibit FXIIa-mediated contact activation, coagulation is not initiated during the arbitrarily defined course of the experiment (30 min). This window of experimentation is sufficiently long to discern samples that are pro- or antithrombotic. Even though coagulation by contact activation can be an unwanted consequence of blood contacting artificial surfaces, our data demonstrate a clear biological dose effect of platelets. This can be important for transfusion medicine, because recent findings on FXII, platelet polyphosphates10, phosphatidylserine distribution22, and platelet microparticles23 have actually revalued contact pathway coagulation as an important contributor to hemostasis, especially in the context of thrombosis24. For instance, the variable yield of platelet transfusions in patients or the variable phosphatidylserine expression of banked platelets may thus induce variability in therapeutic efficacy if successful coagulation is shown to depend on these factors.
By co-immobilizing lipidated rhTF with collagen, the extrinsic coagulation route, or TF pathway, is activated during platelet deposition on collagen. This extends the assay to its most comprehensive mode, as a model for injuries that bring blood into contact with TF-bearing cells and tissue. Our data show that co-immobilization of collagen and TF decreases the moment of coagulation onset but does not alter the rate of coagulation. Of note, the amount of rhTF immobilized to the surface is an important variable in this model25,26 and should be standardized within a given study. In the presence of CTI, the TF pathway can be studied exclusively (not shown) because contact activation is prevented.
In conclusion, our experimental setup combines a model of transfusion by reconstitution of thrombocytopenic blood with banked platelets and a model of hemostasis by calcium-dependent platelet deposition and fibrin formation under hydrodynamic flow. This assay will be used to answer questions on the procoagulant nature of banked platelets and the effects platelet concentrate preparation techniques have on this.
開示
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Foundation for Research and Development of the Belgian Red Cross-Flanders Blood Service. We acknowledge financial support provided by "Bijzonder onderzoeksfond" (BOF – special research fund) from the Ghent University granted to the project with contract number BOF30290744.
Materials
| 1 mL Syringe | BD | A00434 | |
| 10 mL Syringe | BD | 309604 | |
| 2 mL Syringe | BD | 307727 | |
| Alexa Fluor 405 | Life Technologies | A30100 | The manufacturer's manual was followed for labeling of fibrinogen |
| BD vacutainer Eclipse | Becton, Dickinson and Company | 368650 | Blood collection needle with preattached holder |
| BD vacutainer tube with EDTA | Becton, Dickinson and Company | 368856 | |
| BD vacutainer tube with Sodium Citrate | Becton, Dickinson and Company | 366575 | |
| Blocking buffer | in house preparation | in house preparation | 1.0% (w/v) bovine serum albumin and 0.1% (w/v) glucose in HBS |
| Calcein AM | Molecular probes | C1430 | |
| Coagulation buffer | in house preparation | in house preparation | 10mM CaCl2 and 3.75 mM MgCl2 in HBS |
| Conical tube 15mL | Greiner bio-one | 1888271 | |
| Conical tube 50mL | Greiner bio-one | 227261 | |
| Corn trypsin inhibitor (CTI) | Enzyme Research Laboratories | CTI | |
| Denaturated alcohol | Fiers | T0011.5 | |
| DiOC6 | Sigma-Aldrich | 318426 | 3,3′-Dihexyloxacarbocyanine iodide – 1mM solution in DMSO |
| Exigo microfluidic pump | Cellix | EXIGO-PC-FS7.0-MF | |
| Fibrinogen | Sigma-Aldrich | F3879 | Labelled in house with AF 405 – stock concentration 10.7 mg/mL |
| Hematology analyzer | Sysmex | pocH-100i | |
| HEPES buffered saline (HBS) | in house preparation | in house preparation | 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline (0.9% (w/v) NaCl, pH 7.4 |
| Horm Collagen | Takeda/Nycomed | 1130630 | Native equine tendon collagen (type I) Isotonic glucose solution to dilute collagen is supplemented |
| Humidified box | Cellix | HUMID-BOX | |
| Incubation water bath | GFL | 1013 | |
| Microscope | Zeiss | Axio Observer Z1 | equipped with a colibri-LED and high resolution CCD camera |
| Mirus Evo Nanopump | Cellix | 188-MIRUS-PUMP-EVO | with Multiflow8 |
| Pipette | Brand | A03429 | |
| Pipette tips 100-1000 | Greiner bio-one | 740290 | |
| Pipette tips 1-10 | Eppendorf | A08928 | |
| Pipette tips 2-200 | Greiner bio-one | 739280 | |
| Platelet concentrate orbital shaker | Helmer | PF-48i | |
| Precision wipes | Kimtech | 5511 | |
| Pepsin solution | Hanna instrument | HI7073L | 2g pepsin per 75 ml solution, Protein cleaning solution with pepsin |
| Recombinant Human Tissue Factor Innovin | Dade Berhing | B4212-40 | rhTF with synthetic phospholipids |
| Software microscope | Zeiss | N/A | ZEN 2012 |
| Sterile docking device | Terumo BCT | TSCD | |
| Table Top Centrifuge | Eppendorf | 521-0095 | |
| Tube Roller | Ratek | BTR5-12V | |
| Tubing Sealer | Terumo BCT | AC-155 | |
| Vena8 syringe connector pin | Cellix | CONNECTORS-B1IC-PACK100 | |
| Vena8 Fluoro+ Biochips | Cellix | 188V8CF-400-100-02P10 | Coated biochip |
| Vena8 Needles | Cellix | SS-P-B1IC-B1OC-PACK200 | |
| Vena8 Tubing | Cellix | TUBING-TYGON-B1IC-B1OC-ROLL 100F | |
| VenaDelta Y1 Biochips | Cellix | VDY1-400-100-01-02 | Mixing biochip |
| Vortex mixer | VWR | 58816-121 | |
| ZEN2012 software | Zeiss |
参考文献
- Levi, M., Hunt, B. J. A critical appraisal of point-of-care coagulation testing in critically ill patients. J. Thromb. Haemost. 13 (11), 1960-1967 (2015).
- Van Aelst, B., Feys, H. B., Devloo, R., Vandekerckhove, P., Compernolle, V. Microfluidic Flow Chambers Using Reconstituted Blood to Model Hemostasis and Platelet Transfusion In Vitro. J. Vis. Exp. (109), e53823 (2016).
- Witt, S. M., et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat. Commun. 5, 4257 (2014).
- Cazenave, J. P., et al. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol. Biol. 272, 13-28 (2004).
- Roest, M., et al. Flow chamber-based assays to measure thrombus formation in vitro: requirements for standardization. J. Thromb. Haemost. 9 (11), 2322-2324 (2011).
- Zwaal, R. F., Comfurius, P., Bevers, E. M. Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta. 1376 (3), 433-453 (1998).
- Dargaud, Y., Luddington, R., Baglin, T. P. Elimination of contact factor activation improves measurement of platelet-dependent thrombin generation by calibrated automated thrombography at low-concentration tissue factor. J. Thromb. Haemost. 4 (5), 1160-1161 (2006).
- Bevers, E. M., et al. Defective Ca(2+)-induced microvesiculation and deficient expression of procoagulant activity in erythrocytes from a patient with a bleeding disorder: a study of the red blood cells of Scott syndrome. Blood. 79 (2), 380-388 (1992).
- Bevers, E. M., Comfurius, P., van Rijn, J. L., Hemker, H. C., Zwaal, R. F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 122 (2), 429-436 (1982).
- Muller, F., et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 139 (6), 1143-1156 (2009).
- Smith, S. A., et al. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. U.S.A. 103 (4), 903-908 (2006).
- Tiberghien, P., Follea, G., Muller, J. Y. Platelet Transfusions in Acute Leukemia. N. Engl. J. Med. 375 (1), 96-97 (2016).
- Deckmyn, H., Feys, H. B. Assays for quality control of platelets for transfusion. ISBT Science Series. 8 (1), 221-224 (2013).
- Sakariassen, K. S., Bolhuis, P. A., Sixma, J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 279 (5714), 636-638 (1979).
- Alevriadou, B. R., et al. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood. 81 (5), 1263-1276 (1993).
- Nesbitt, W. S., et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15 (6), 665-673 (2009).
- Stalker, T. J., et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 121 (10), 1875-1885 (2013).
- Swieringa, F., Kuijpers, M. J., Lamers, M. M., vander Meijden, P. E., Heemskerk, J. W. Rate-limiting roles of the tenase complex of factors VIII and IX in platelet procoagulant activity and formation of platelet-fibrin thrombi under flow. Haematologica. 100 (6), 748-756 (2015).
- McDonald, J. C., et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis. 21 (1), 27-40 (2000).
- Gutierrez, E., et al. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab. Chip. 8 (9), 1486-1495 (2008).
- Hartman, R. L., McMullen, J. P., Jensen, K. F. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. Engl. 50 (33), 7502-7519 (2011).
- Podoplelova, N. A., et al. . Blood coagulation factors bound to procoagulant platelets are concentrated in their cap structures to promote clotting. , (2016).
- Der Meijden, P. E. V. a. n., et al. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 10 (7), 1355-1362 (2012).
- Nickel, K. F., et al. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood. 126 (11), 1379-1389 (2015).
- Okorie, U. M., Denney, W. S., Chatterjee, M. S., Neeves, K. B., Diamond, S. L. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 111 (7), 3507-3513 (2008).
- Shen, F., Kastrup, C. J., Liu, Y., Ismagilov, R. F. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler. Thromb. Vasc. Biol. 28 (11), 2035-2041 (2008).

