Hydroponics: A Versatile System to Study Nutrient Allocation and Plant Responses to Nutrient Availability and Exposure to Toxic Elements
概要
Here, we present an easy-to-follow protocol to establish a successful hydroponic system for plant nutrition studies. This protocol has been extensively tested in Arabidopsis and can easily be adapted to other plant species to study specific nutritional requirements or the effect of non-essential elements on plant growth and development.
Abstract
Hydroponic systems have been utilized as one of the standard methods for plant biology research and are also used in commercial production for several crops, including lettuce and tomato. Within the plant research community, numerous hydroponic systems have been designed to study plant responses to biotic and abiotic stresses. Here we present a hydroponic protocol that can be easily implemented in laboratories interested in pursuing studies on plant mineral nutrition.
This protocol describes the hydroponic system set up in detail and the preparation of plant material for successful experiments. Most of the materials described in this protocol can be found outside scientific supply companies, making the set up for hydroponic experiments less expensive and convenient.
The use of a hydroponic growth system is most advantageous in situations where the nutrient media need to be well controlled and when intact roots need to be harvested for downstream applications. We also demonstrate how nutrient concentrations can be modified to induce plant responses to both essential nutrients and toxic non-essential elements.
Introduction
Plants are among the few organisms that can synthesize all the required metabolites from inorganic ions, water and CO2 using the energy captured from the sun1. Hydroponics is a method of growing plants that takes advantage of this fact by providing all of the nutrients, in their inorganic form, in a liquid solution with or without solid media. Hydroponic systems have been extensively used by scientists for exploring nutrient requirements and also the toxicity of some elements in Arabidopsis and other plant species 2-5. For instance, Berezin et al.3, Conn et al.4, and Alatorre-Cobos et al.2 used hydroponic systems and several plant species including tomato and tobacco, to generate sufficient plant biomass for mineral analysis2-4. Industrial applications of hydroponics have also been developed for crops such as tomato and lettuce6. Here, we outline the use of hydroponics in the context of research, possible variations in available methods, and finally present a system that can be easily scalable and useful for research laboratories interested in studying plant mineral nutrition.
Hydroponic systems allow for easy separation of root tissue and precise control of nutrient availability
Hydroponics offers several advantages over soil-based systems. When removed from soil, root tissue is often mechanically sheared causing loss of tissue or damage. This is particularly true for fine root structures such as lateral roots and root hairs. Hydroponic systems that do not utilize an inert particulate media allow a less invasive separation of root and shoot tissues.
In soil systems, nutrient bioavailability changes throughout the soil matrix as nutrients bind to soil particles creating micro-environments within the soil. This heterogeneity could add an extra level of complexity in experiments needing a precise control on the external concentration of nutrients or other molecules. In contrast, the hydroponic solution is homogeneous and can be easily replaced throughout the course of the experiment.
Variants of hydroponic systems
All hydroponic cultures rely on a nutrient solution to deliver essential elements to the plant. In addition to the nutrients, the roots also need a steady supply of oxygen. When roots become anoxic they are unable to take up and transport metabolites to the rest of the plant body7. Hydroponic systems can be classified based on how they deliver oxygen and other nutrients to the roots: oxygen delivery by saturating the solution with air (classical hydroponics), by not submerging the roots at all times, or by allowing the roots to be completely exposed to the air (aeroponics)8. In hydroponics, nutrient solution can be saturated with air prior to its use and changed frequently, or air can be continuously supplied in the solution over the life cycle of the plant9. Alternatively, plants may also be grown on inert media (e.g., rockwool, vermiculite, or clay pellets) and subjected to wet-dry cycles by dripping solution through the media or periodically submerging the substrate in the nutrient solution10. In aeroponics, roots are sprayed with the nutrient solution to prevent desiccation.
Disadvantages of hydroponic systems
Although hydroponic cultures offer clear advantages over soil-based systems, there are some considerations that must be acknowledged when interpreting the data. For instance, hydroponic systems expose plants to conditions that may be seen as non-physiological. Therefore, phenotypes or plant responses detected using hydroponic systems may vary in magnitude when plants are grown in alternative systems (e.g., soil or agar-based media). These considerations are not unique for hydroponic systems; differential responses can also be observed if plants are grown in different types of soil 11,12.
The following protocol provides step-by-step instructions on how to set up a hydroponic system in a laboratory. This protocol has been optimized for Arabidopsis thaliana (Arabidopsis); however, similar or in some cases identical steps can be used to grow other species.
Protocol
1. Seedling Nursery
- Vapor-phase sterilization of Arabidopsis seeds
- Pour seeds (40-50 mg) into 1.5 ml centrifuge tubes. (See Figure 1 for appropriate seed volume, ~ 50 µl). Label each tube with pencil (ink may fade away during sterilization). Place each labeled tube, cap open, into a desiccator13.
- Place the desiccator in an active fume hood and close the desiccator's valve.
- Aliquot 100 ml of bleach (NaClO 6.15%) into a 250 ml beaker and then place it in the desiccator.
- Quickly add 3 ml of 12 M hydrochloric acid to the bleach using a transfer pipette. Quickly close the lid of the desiccator as the reaction proceeds rapidly. Allow the sterilization to proceed for 4 hr (marking a tube with ink and seeing the ink fade away helps to visualize that a sufficient amount of chlorine gas has been generated).
CAUTION: Chlorine gas is toxic; handle its residues with extra safety precautions in a functional fume hood. Contact local authorities or visit the webpage of the Environmental Health and Safety Department – University of Missouri (ESH-MU)14 for chemical safety and guidelines for using a fume hood: https://ehs.missouri.edu/chem/. - Fifteen minutes before sterilization is complete (3.75 hr), turn on a laminar flow hood and clean the surface using 70% ethanol.
- After 4 hr of sterilization open the valve, briefly remove the lid of the desiccator inside of the fume hood, remove the bleach, and dispose it according to institutional procedures. This step will release a large portion of the chlorine fumes. Seal the sterilization chamber and bring it to the laminar flow hood. Open the lid widely and aerate the sterilized seeds for approximately 40 min. After this time, use the seeds immediately or store in a dry place.
Note: Vapor-phase sterilization of seeds is recommended but other methods such as alternate washes with ethanol, bleach and water as described in Alatorre-Cobos et al.2 are equally efficient.
- Culture media for seeds germination
Note: The culture media prepared in this step is ¼ Murashige and Skoog (MS) with vitamins15.- Add 450 ml deionized water (DI water), 0.55 g MS media plus vitamins, 0.3 g MES (4-morpholineethanesulfonic acid hydrate), and a magnetic stir bar into a 1 L glass beaker.
- Dissolve and adjust pH to 5.7 using NaOH and then add 3.5 g phytoagar. Keep stirring the solution for 5 more minutes.
- Pour the whole solution into a graduated cylinder and add DI water up to 500 ml. Autoclave this 500 ml solution, with the magnetic stir bar inside, using a 1 L autoclavable bottle.
- After the solution has been autoclaved, stir the solution for 7-10 min using the magnetic stirrer in the bottle.
- After the media has cooled down to 50-60 °C, pour the media into plates under sterile conditions and let it solidify. Plates can be stored for later use in the cold-room.
- Seed plating
- Turn on the laminar flow hood 15 min prior to use and clean the surface with 70% ethanol. The following items are required: sterile seeds, filter paper, toothpicks, micropore tape and ¼ MS plates.
- Place the sterile seeds on a sterile filter paper. Slightly wet one end of a sterile toothpick (with sterile water or by poking the ¼ MS media). Use this moisturized end to pick the seeds from the filter paper and then lay them onto the media surface.
- Spread the seeds across the plate at a density of approximately 1 seed per cm2 (Figure 2). Then use micropore tape to keep the plate lid attached to plate body. This type of tape helps to prevent contamination while allowing gas exchange between the air and the microclimate inside the plate.
- Before germination, stratify seeds by keeping the plates two days in the cold room covered from light.
- After stratification, place the seeds in a growth chamber or in a place with optimal growth conditions (23 °C, 16 hr light/8 hr dark and 60% relative humidity for Arabidopsis). Seedlings will be ready for hydroponics 10-12 days after germination.
Note: During germination there may be significant condensation under the lid of the plate, to prevent drowning, the excess water should be discarded under sterile conditions in a laminar flow hood.
2. Hydroponic Setup and Transplant Process
- Hydroponic solution
Note: As mentioned in the introduction, plants may have specific nutritional requirements. Arabidopsis has been successfully grown with the nutrient solution shown in Table 116. Depending on the suppliers, the salts listed here may have different water content (hydrated) and using such alternatives does not affect the properties of the nutrient solution as long as the molarity is held constant.- Prepare the stock solutions of each macronutrient in different bottles (Table 1) and all micronutrients except Fe-EDTA in a sterile bottle (sterilize by filtration using 0.22 µm membranes). Always add Fe-EDTA at last when mixing the solution. Prepare a 10x nutrient solution in advance of the experiment but autoclave and store at 4 °C. Use or change the nutrients only when the nutrient solution has reached room temperature.
- Transplanting
- Prepare plant holder and hydroponic containers
- Make an incision in the foam, running along its length using a razor blade (see Figure 3). Prepare one plug per plant.
- Liquid-autoclave foam tube plugs soaked in DI water.
- Cut the foam panel into smaller boards, making sure that the width and length of foam boards are 0.5-1.0 cm less than size of the container (see Figure 4).
- Use a cork borer to create holes on the foam board. The density of the plants should be evenly distributed, ideally 1 plant per 10 cm2. This density will keep plants neatly separated from each other; higher densities however are possible and will not preclude the success of the experiments. Make sure the size of the holes matches the size of the plugs (see Figure 4).
- Fill the containers with the nutrient solution. Make sure that the depth of the solution is enough for root development (at least 5 cm). Then carefully place the foam boards onto the solution's surface.
- Set up the air-pump system to provide oxygen into the solution (see Figure 5).
Note: Fill in the hydroponic container with nutrient solution the same day seedlings are being transplanted. Covering the sides of the container from light will help to prevent algal growth.
- Seedling transfer from plates to hydroponic system
- Use small tweezers to gently pull each seedling out of the medium plate and lay the root along the incision of the foam tube plug. Carefully plug the foam tube holding the seedling into the foam board then place the board back to the hydroponic container. See Figure 6 for appropriate manipulation.
- Prepare plant holder and hydroponic containers
3. Hydroponic Experiments
- Nutrient solution replacement and manipulation
- Nutrient solution replacement
- To replace the nutrient solution, prepare fresh hydroponic solution as described in step 2.1. Remove the foam board containing plants from the hydroponic container and place it in a temporary container filled with water or hydroponic solution.
- Discard the old solution, rinse the container briefly three times with DI water. Add the freshly prepared hydroponic solution into this container and gently place the foam board with plants back into the hydroponic container. Replace the hydroponic solution twice a week.
- Changing the nutrient composition of the hydroponic solution
- Adjust the composition of the hydroponic solution shown in Table 1 to modify the final concentration of an element of interest. For example, to induce iron (Fe) deficiency, modify the hydroponic solution to decrease the concentration of Fe-EDTA. Include a set of control plants grown on full (or replete) hydroponic solution, without any modification, for comparison.
- To manipulate the nutrient solution with a toxic element, first prepare an independent stock solution of the desired toxic element, preferably 1,000x concentrated. Use a pipette to spike the hydroponic solution with the toxic element at the desired final concentration using the 1,000x concentrated stock.
- For example, in order to make 3 L of hydroponic solution containing 20 µM of cadmium, prepare a 0.5 M CdCl2 stock, and add 120 µl of the 0.5 M CdCl2 stock into the 3 L hydroponic solution. Include a control set of plants grown on hydroponics without CdCl2 for comparison.
CAUTION: Toxic elements such as cadmium, arsenic and lead are very dangerous for human health and the environment. Please contact local authorities or visit the webpage of the EHS-MU (https://ehs.missouri.edu/train/chemical.html)14 for environmental and health safety guidelines prior to conducting experiments.
- Nutrient solution replacement
- Instrument sterilization for next experiments
- As almost all the material used to prepare the hydroponic set up can be reused, clean the different parts with diluted bleach (NaClO 0.6%).
- After rinsing with bleach, rinse all materials thoroughly with DI water. Keep containers, foam boards, and aquarium bubble stones in a dry place for future use. Foam plugs are ready for reuse after removing the roots and being autoclaved.
Representative Results
In this section, the results of two types of experiments, using the hydroponic system described here, are presented. In the first experiment, the nutrient solution was modified to obtain different concentrations of zinc. We also modified the nutrient solution by adding non-lethal concentrations of the toxic element cadmium (Figure 7). In the second experiment, we used inductively coupled plasma optical emission spectrometry (ICP-OES) 1 to measure the elemental composition of roots and leaves of plants grown in the hydroponic solution containing cadmium (Figure 8). This experiment illustrates the advantages of obtaining roots and leaves separately.
Experiment 1
Arabidopsis seedlings (Col-0) were grown in the hydroponic system described in protocol steps 1 and 2. Plants were allowed to grow for a total of 3 weeks before being treated with different zinc concentrations (Figure 7A-B) or a non-lethal concentration of cadmium (Figure 7C). Six days post treatment, plants grown at high zinc concentrations (> 42 µM) showed delayed growth due to Zn toxicity, while plants without extra zinc added also show delayed growth compared to plants grown with 7 µM Zn2+. Figure 7 also shows the reduction in shoot growth, root growth, and chlorotic leaf symptoms typical of plants exposed to cadmium (Figure 7C).
Experiment 2
Col-0 plants were grown as described in steps 1 and 2. After two weeks, the non-modified (replete) solution was replaced with 80 ml of hydroponic solution containing 20 µM Cd. After 72 hours, root tissues were washed by transferring the whole foam board with plants to a new vessel containing 80 ml of Tris 20 mM (pH 8.0) and 5 mM EDTA. This solution will remove the heavy metals bound to the surface of the root. Plants were incubated in the EDTA-containing solution on a rotary shaker for 5 minutes. EDTA solution was then replaced by 80 ml of DI water and plants were incubated on the rotary shaker for an additional 5 minutes. This rinsing step with DI water was repeated twice. After rinsing the plants with DI water, leaf and root tissues were harvested independently and processed for ICP-OES1. Figure 8 shows that the elemental composition of leaves is different from roots, where macronutrients (Ca, K, and Mg) in leaf tissue are present in higher concentration compared to roots. On the other hand, micronutrients such as Zn and Fe are preferentially accumulated in roots. The concentration of the non-essential element cadmium was found to be higher in roots compared to shoots.
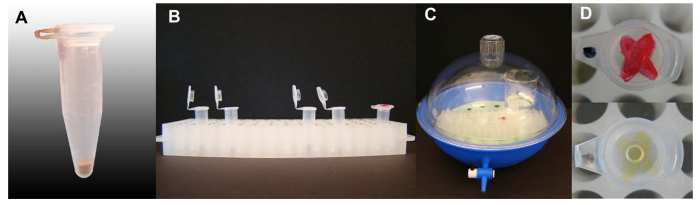
Figure 1. Vapor-phase sterilization of Arabidopsis seeds. (A) Amount of Arabidopsis seeds per 1.5 ml centrifuge tubes. (B) Tubes containing seeds with caps open in the tube rack holder ready for sterilization, one tube with an ink-marked on the cap is included. (C) Sterilization set up inside a desiccator, lid and valve closed. (D) The ink-mark on the lid of a tube included in the seed sterilization process with strong color of ink-mark before and after sterilization. Please click here to view a larger version of this figure.
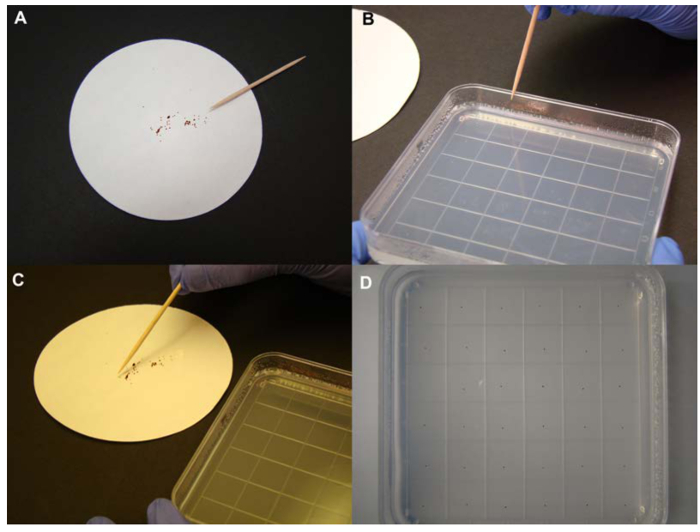
Figure 2. Seed plating step. (A) Seeds are placed on sterilized paper before plating. A sterilized toothpick is also required for this step. (B) Slightly wet the end of the toothpick with the media or water on the side of the medium plate. (C) Seeds are moved to ¼ MS plates. (D) An ideal density of seeds is ≈1 seed/cm2. Please click here to view a larger version of this figure.
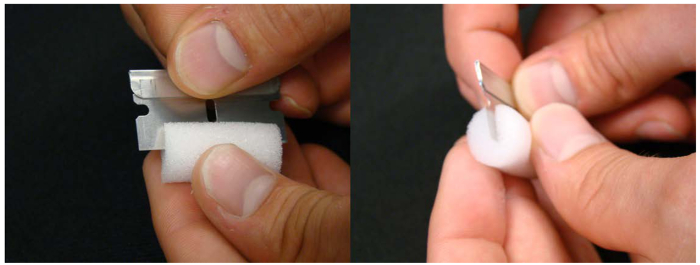
Figure 3. Foam plug used to hold seedlings in the nutrient solution. An incision on half of the foam tube plug helps holding the seedling during transplanting from plates to hydroponics. Please click here to view a larger version of this figure.
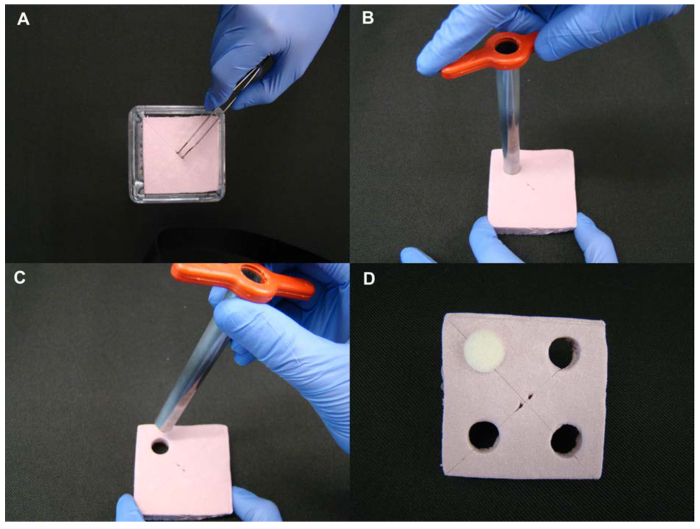
Figure 4. Foam board preparation. (A) Check the size of the template foam board with the container size before preparing foam boards in large quantities. Two small perforations made at center of the foam board make it is easier to hold and handle the foam using tweezers. (B-C) A cork borer is used to create holes on the foam board. (D) Check the proper fit between the foam tube plug and holes created on the foam board. Please click here to view a larger version of this figure.
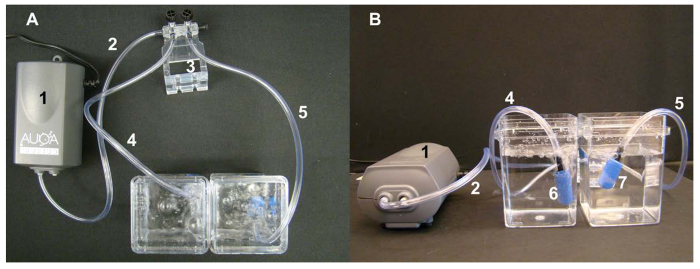
Figure 5. Air-pump setting for hydroponic experiment from top-view (A) and side-view (B). The numbers indicate: 1 – pump supplying air; 2 – plastic tubing connecting the air pump with the valve system to control the air-flow; 3 – the valve system; 4 and 5 – plastic tubing connecting the valve system with bubble stones for aeration; 6 and 7 – bubble stones (sold for fish tanks). Please click here to view a larger version of this figure.

Figure 6. Transferring seedlings to the hydroponic system. (A) Use tweezers to take a seedling out of the medium plate. (B) Place the seedling root along the incision on the foam tube plug. (C) Insert the foam tube plug into the foam board. (D) A completed foam board setting with seedlings ready to be placed on the nutrient solution. Please click here to view a larger version of this figure.
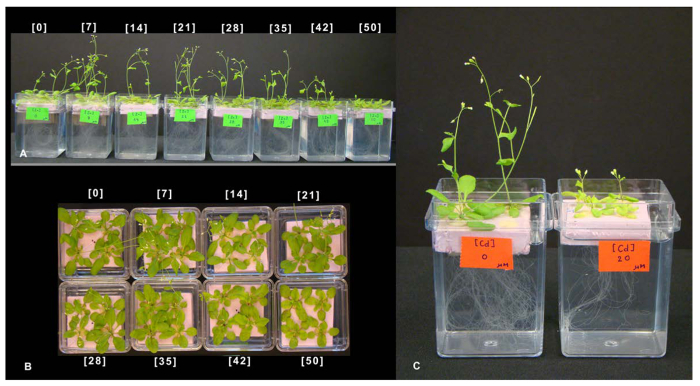
Figure 7. Nutrient solutions can be modified to test deficiency or toxic effects of elements. 4 week-old hydroponically grown Arabidopsis 6 days after treatment: (A-B) plants grown with 0, 7, 14, 21, 28, 35, 42, and 50 µM of Zn. Plants grown at high Zn concentrations (> 42 µM) show delayed growth (toxicity) while plants without Zn added also show delayed growth (nutrient deficiency) compared to plants grown with 7 µM Zn2+. (C) Plants grown in the absence (left) or presence of 20 µM Cd in the nutrient solution (picture was taken after 6 days of Cd exposure). Cadmium exposure induces chlorosis and reduces growth. Please click here to view a larger version of this figure.
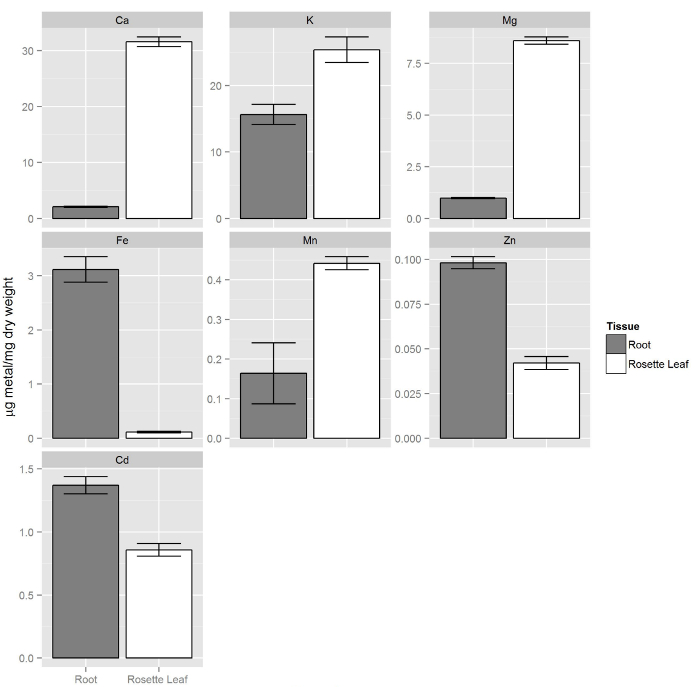
Figure 8. Elemental composition of roots and shoots from plants grown hydroponically. Shoots contain more macronutrients (Ca, K, Mg) compared to roots while the essential micronutrients zinc and iron are more concentrated in roots. Similarly the non-essential element cadmium is preferentially accumulated in roots. Error bars represent the 95% confidence intervals (n = 14, shoots and n= 9, roots). Please click here to view a larger version of this figure.
| Type of nutrient | Salt/Reagent | Concentration in hydroponic solution | Unit |
| Macronutrient | KNO3 | 1.250 | mM |
| Macronutrient | KH2PO4 | 0.625 | mM |
| Macronutrient | MgSO4 | 0.500 | mM |
| Macronutrient | Ca(NO3)2 | 0.500 | mM |
| Micronutrient | H3BO3 | 17.500 | µM |
| Micronutrient | MnCl2 | 5.500 | µM |
| Micronutrient | ZnSO4 | 0.500 | µM |
| Micronutrient | Na2MoO4 | 0.062 | µM |
| Micronutrient | NaCl2 | 2.500 | µM |
| Micronutrient | CoCl2 | 0.004 | µM |
| Micronutrient | FeEDTA | 12.500 | µM |
Table 1. Effective concentration of nutrients in the hydroponic solution.
Discussion
The health of seedlings used for hydroponics is one of the major factors contributing to the success of a hydroponic experiment. Sterilization of instruments, seeds, and culture media also play an important role in reducing the risk of contamination and provide a good start for the plants before they are transplanted into the hydroponic system. A working environment with facilities such as an autoclave, fume hood, cold-room (4 °C), and growth space with controlled conditions (light intensity and temperature) is necessary for a good experimental set up.
The freshness of the nutrient solution also determines the plant health and in turn determines the success of a hydroponic experiment. Since water evaporates faster under direct lighting, the concentration of salts will change due to a reduction of total solution volume; therefore it is best to change the hydroponic solution at least twice a week. However, if large, deep containers equipped with an air pump system are used it may not be necessary to replace the nutrient solution for experiments that are short in duration. Note that in the case of Arabidopsis we used Magenta vessels (77 mm width x 77 mm length x 97 mm height) but other, larger containers can also be used to accommodate larger plants.
For researchers interested in plant nutrients, hydroponic experiments provide a unique setting to test plant phenotypes and responses to different nutrient availability17. By manipulating the concentrations of the elements of interest, researchers can set up different experiments to test the effects of sufficiency, deficiency, or toxic concentrations of essential and non-essential nutrients. Compared to the soil-based system, the hydroponic system provides a more homogeneous nutrient medium to the plants with less risk of soil-borne diseases. In addition, both root and shoot tissues can be harvested and separated easily for further analyses on specific plant tissues.
In the representative section, we introduced two examples in which a simple hydroponic system was used for more detailed studies on plant nutrition. In the first example, by growing plants on a zinc concentration gradient, we were able to illustrate the level of control that can be achieved on nutrient composition using this hydroponic system. Plants grown with 7 µM Zn grew much more vigorously compared to plants grown in 50 µM Zn, while plants grown without extra Zn added were stunted compared to plants grown with 7 µM Zn. This was in part due to the length of time the plants were allowed to grow under sufficient conditions; earlier removal of Zn from the media is likely to induce stronger zinc-deficiency symptoms. Applying the same principle, we were able to induce toxicity using the non-essential metal, cadmium, which is known to impair plant growth.
In the second example, the elemental composition of Col-0 roots and shoots treated with 20 µM Cd for 72 hr was determined by ICP-OES. We found differences in all detected metals between roots and shoots. Macro-elements were found in higher concentrations in the shoots relative to the roots, while iron and zinc were found more abundant in roots. Cadmium followed a pattern similar to iron and zinc, being more concentrated in roots compared to shoots. These data reinforce the idea that leaves and roots provide different information about the ionome status of the plant and therefore both tissues need to be analyzed separately to understand mineral nutrition and composition at the whole plant level. Besides ICP-OES several spectroscopic methods such as Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma Mass Spectrometry (ICP-MS) can also be used to measure the elemental composition (ionome) of plant tissues18-20.
In a hydroponic experiment, the symptoms and phenotypes of plants responding to different nutrient conditions represent the beginning of what could be extended into more elaborated analyses such as gene expression (transcriptomics) and protein abundance (proteomics). These -omic techniques are keys to integrate plant metabolism by considering processes in a tissue-specific manner.
開示
The authors have nothing to disclose.
Acknowledgements
This research was supported by the University of Missouri Research Board (Project CB000519) and the US National Science Foundation (IIA-1430428 to DMC). Nga T. Nguyen was supported by the Vietnam Education Foundation Training Program (Exchange visitor program No. G-3-10180). We also thank Roger Meissen (MU Bond Life Sciences Center) for his assistance and expertise during the video recording and editing sessions.
Materials
| For seed sterilization | |||
| Bleach | The Clorox Company | NA | The regular bleach |
| www.cloroxprofessional.com | |||
| Hydrochloric acid | Fisher Scientific | A144-500 | |
| Desiccator body | Nalgene | D2797 SIGMA | Marketed by Sigma-Aldrich |
| Desiccator plate | Nalgene | 5312-0230 | Marketed by Thermo Scientific |
| For one quarter MS medium preparation | |||
| MES | Acros Organics | 172591000 | 4-Morpholineethanesulfonic acid hydrate |
| Murashige and Skoog (MS) | Sigma-Aldrich | M0404-10L | |
| KOH | Fisher Scientific | P250-500 | |
| Phytoagar | Duchefa Biochemie | P1003.1000 | |
| Square plate | Fisher Scientific | 0875711A | Disposable Petri Dish With Grid |
| For seed plating | |||
| Filter paper | Whatman | 1004090 | |
| Toothpick | Jarden Home Brands | NA | |
| Aluminum foil | Reynolds Wrap | NA | Standard aluminum foil |
| Micropore tape | 3M Health Care | 19-898-074 | Surgical tape; Marketed by Fisher Scientific |
| For hydroponic solution preparation | |||
| KNO3 | Fisher Scientific | BP368-500 | |
| KH2PO4 | Fisher Scientific | P386-500 | |
| MgSO4 | Fisher Scientific | M63-500 | |
| Ca(NO3)2 | Acros Organics | A0314209 | |
| H3BO3 | Sigma | B9645-500G | |
| MnCl2 | Sigma-Aldrich | M7634-100G | |
| ZnSO4 | Sigma | Z0251-100G | |
| Na2MoO4 | Aldrich | 737-860-5G | |
| NaCl2 | Fisher Scientific | S271-1 | |
| CoCl | Sigma-Aldrich | 232696-5G | |
| FeEDTA | Sigma | E6760-100G | |
| “Stericup & Steritop” bottle | Milipore Corporation | SCGVU02RE | Micronutrient container |
| For root wash buffer preparation | www.milipore.com | ||
| EDTA | Acros Organics | A0305456 | |
| Tris | Fisher Scientific | BP154-1 | |
| For hydroponic set up | |||
| Autoclavable foam tube plug | Jaece Industries Inc. | L800-A | Identi-Plugs fit to holes with 2R=6-13mm |
| Foam Board | Styrofoam Brand Dow | ESR-2142 | Thickness is 1/2 inches |
| Cork borer | Humboldt | H-9662 | Cork Borer Sets with Handles, , Plated Brass Set of 6, 3/16" to 1/2" OD Size |
| Air pump | Aqua Culture | MK-1504 | |
| Marketed by Wal-mart Stores, Inc. | |||
| Airline tubing and aquarium bubble stones | Aqua Culture | Tubing: 928/25-S | |
| Marketed by Wal-mart Stores, Inc. | Stone: ASC-1 | ||
| その他 | |||
| Ethanol | Fisher Scientific | A995-4 | Reagent Alcohol |
| Cadmium Chloride (CdCl2) | Sigma-Aldrich | 10108-64-2 | |
参考文献
- McDowell, S. C., et al. Elemental Concentrations in the Seed of Mutants and Natural Variants of Arabidopsis thaliana Grown under Varying Soil Conditions. PLoS ONE. 8, 1-11 (2013).
- Alatorre-Cobos, F., et al. An improved, low-cost, hydroponic system for growing Arabidopsis and other plant species under aseptic conditions. BMC Plant Biol. 14, 69-69 (2014).
- Berezin, I., Elazar, M., Gaash, R., Avramov-Mor, M., Shaul, O., Asao, T. The Use of Hydroponic Growth Systems to Study the Root and Shoot Ionome of Arabidopsis thaliana. Hydroponics – A Standard Methodology for Plant Biological Researches. , (2012).
- Conn, S. J., et al. Protocol: optimising hydroponic growth systems for nutritional and physiological analysis of Arabidopsis thaliana and other plants. Plant Methods. 9, 4-4 (2013).
- Kopittke, P. M., Blamey, F. P. C., Asher, C. J., Menzies, N. W. Trace metal phytotoxicity in solution culture: a review. J. Exp. Bot. 61, 945-954 (2009).
- Gent, M. P. N. Composition of hydroponic lettuce: effect of time of day, plant size, and season. J. Sci. Food Agric. 92, 542-550 (2012).
- Gibbs, J., Turner, D. W., Armstrong, W., Darwent, M. J., Greenway, H. Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Funct. Plant Biol. 25, 745-758 (1998).
- Zobel, R. W., Del Tredici, P., Torrey, J. G. Method for Growing Plants Aeroponically. Plant Physiol. 57, 344-346 (1976).
- Chang, D. C., Park, C. S., Kim, S. Y., Lee, Y. B. Growth and Tuberization of Hydroponically Grown Potatoes. Potato Research. 55, 69-81 (2012).
- Resh, H. M. . Hydroponic Food Production : A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, Seventh Edition. , 199-292 (2012).
- Sharma, H. K., Chawan, D. D., Daiya, K. S. Effect of different soil types on plant growth, leaf pigments and sennoside content in Cassia species. Pharmaceutisch weekblad. 2, 65-67 (1980).
- Strojny, Z., Nowak, J. S. Effect of different growing media on the growth of some bedding plants. Acta horticulturae. 19, 157-162 (2004).
- Bent, A. Arabidopsis thaliana floral dip transformation method. Methods Mol Biol. 2, 87-103 (2006).
- . Chemical Safety Environmental Health and Safety – University of Missouri Available from: https://ehs.missouri.edu/ (2013)
- Murashige, T., Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 15, 473-497 (1962).
- Lee, D. A., Chen, A., Schroeder, J. I. ars1, an Arabidopsis mutant exhibiting increased tolerance to arsenate and increased phosphate uptake. Plant J. 35, 637-646 (2003).
- Pii, Y., Cesco, S., Mimmo, T. Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol. Biochem. 94, 48-56 (2015).
- Baxter, I. Ionomics: studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 12, 381-386 (2009).
- Baxter, I. Ionomics: The functional genomics of elements. Brief Funct Genomics. 9, 149-156 (2010).
- Salt, D. E. Update on plant ionomics. Plant Physiol. 136, 2451-2456 (2004).

