A Protocol for the Use of Remotely-Supervised Transcranial Direct Current Stimulation (tDCS) in Multiple Sclerosis (MS)
概要
The goal of this pilot study is to describe a protocol for the remotely-supervised delivery of transcranial direct current stimulation (tDCS) so that the procedure maintains standards of in-clinic practice, including safety, reproducibility, and tolerability. The feasibility of this protocol was tested in participants with multiple sclerosis (MS).
Abstract
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that uses low amplitude direct currents to alter cortical excitability. With well-established safety and tolerability, tDCS has been found to have the potential to ameliorate symptoms such as depression and pain in a range of conditions as well as to enhance outcomes of cognitive and physical training. However, effects are cumulative, requiring treatments that can span weeks or months and frequent, repeated visits to the clinic. The cost in terms of time and travel is often prohibitive for many participants, and ultimately limits real-world access.
Following guidelines for remote tDCS application, we propose a protocol that would allow remote (in-home) participation that uses specially-designed devices for supervised use with materials modified for patient use, and real-time monitoring through a telemedicine video conferencing platform. We have developed structured training procedures and clear, detailed instructional materials to allow for self- or proxy-administration while supervised remotely in real-time. The protocol is designed to have a series of checkpoints, addressing attendance and tolerability of the session, to be met in order to continue to the next step. The feasibility of this protocol was then piloted for clinical use in an open label study of remotely-supervised tDCS in multiple sclerosis (MS). This protocol can be widely used for clinical study of tDCS.
Introduction
tDCS is a relatively recent therapy that operates through the use of low amplitude (2.0 mA or less) direct current to modulate cortical excitability 1. Hundreds of clinical trials have demonstrated tDCS to be safe and well-tolerated2-4. tDCS is easier to use, lower in cost, and better tolerated when compared to other methods such as transcranial magnetic stimulation (e.g., tDCS has not been associated with the development of seizures 5,6). Multiple tDCS sessions are required for benefit, especially when administered with the goal of enhancing rehabilitation outcomes.7-10
It is not yet known how many tDCS sessions are necessary or optimal, but the effects are cumulative with little evidence that tDCS over a single session produces behaviorally meaningful changes.2,11 For example, studies of depression have found 30 or more sessions needed for full benefit in some participants. 12,13 Multiple sessions are especially important when pairing tDCS with a behavioral therapy, which only occurs with rigorous repetition across many sessions. 14
For many patients and caregivers, traveling to the outpatient facility to receive repeated tDCS treatment sessions is a major obstacle in terms of time, cost and travel arrangements. This real-world limitation has resulted in studies with small sample sizes and without adequate power or design to draw conclusions that can lead to clinical use.15 Remote tDCS delivery would allow for participation in study protocols from home or other locations, and reach those patients who otherwise would not have access to these trials. Further, it allows the possibility for testing “on-demand” application for indications such as epilepsy and migraines.
We have worked with a diverse group of clinical investigators interested in remotely-supervised tDCS to develop guidelines and standards for remotely-supervised tDCS delivery including specialized equipment and specific training requirements both for staff and study participants16. Here, we developed a protocol to follow these guidelines and test for feasibility in patients with multiple sclerosis (MS), a disorder where tDCS may be a useful tool for the management of its symptoms. 11,17-23
Protocol
Ethics Statement: Stony Brook University Institutional Review Board (IRB) approved this protocol on February 10, 2015.
1. Recruitment of Participants for Remotely-Supervised tDCS
- Provide recruited study participants with information on the device and the ten day study protocol.
2. Inclusion/Exclusion Criteria
- Health
- Ensure that a clinician has provided medical clearance for all enrolled participants at the baseline visit.
- tDCS tolerability and laptop aptitude
- Ensure that participants are familiar with the basic functioning of a laptop computer and how to connect the computer to Wi-Fi internet. For both the use of the laptop, and the setup of the tDCS device, ensure that participants (or a proxy), have the dexterity to complete the procedures.
- Beyond setup, at baseline visits, give participants a 1 min trial dose of 1.5 mA of tDCS to determine tolerability. If participants cannot tolerate this session, a lower dose of 1.0 mA can be applied. If neither is tolerated, exclude the participant.
- Environmental
- Confirm that participants have access to a distraction-free, well lit, clean environment with a safe area to store the device and device kit.
- Compliance
- If a participant does not adhere to the scheduled video conference session in a timely fashion, exclude that participant.
- Stop Criteria
- At each participant interaction, review a series of predetermined "stop criteria" to ensure that participants tolerate the session, adhere to the study schedule, and safely operate the device without adverse effects. Should any of these be met, exclude the participant from the study (Figure 1).
3. Materials
Note: As recommended in the published guidelines for remotely-supervised use16, precise electrode preparation and placement must mirror the clinic protocol with respect to dose control and ongoing monitoring.
- Device Kit
- Prepare the same materials for every remote tDCS participant. This includes a study provided laptop computer with secure video conferencing software, the device, and a device kit.
- Design the device kit for ease of use and organize it by day (Figure 2).
- Prepare the kit with twenty wrapped sponge pockets of size 5x5cm with a perforation at the top (two per day required for a 10-day study) , twenty syringes pre-filled with 6 mL of saline solution for each sponge pocket, a wash bottle filled with extra saline, spare batteries for the device, and a handheld mirror for headset application.
- In addition, require that study users have a portable home phone or mobile phone at their workspace for each session.
- Device
- Choose a tDCS device that only releases a session through a one-time use unlock code. Ensure that the study device has functionality whereby participants can only administer a session after being provided the code by the study technician. In addition, ensure that in the selection of a study device, contact quality is continually monitored to ensure safe and proper use.
- Video Monitoring
- Choose a secure video conferencing software to allow for remote supervision of the proper setup of the device, optimal contact quality, and that the intended user is applying the session.
- Use the video conferencing software as another method of training. Instruct users how to turn the device on, read their contact quality, troubleshoot poor contact quality, enter a one-time use unlock code, and safely remove the device after shutdown.
- Headgear
- Design the headgear for simple and robust electrode positioning. Use a single-position headgear to eliminate room for user error, and to help conserve the placement of the montage. Configure a cap-like design, with clearly labeled sponge markers to enhance the reliability of placement in the single-position headgear with the anodal electrode placed on the left side.
4. Training
Note: Complete the majority of participant training during the first baseline session of the remote study. Roughly 1-2 hr of the baseline visit should be spent on training. Allow participants to view an instructional tDCS video as their first instruction.
- Instructional video
- Set up the instructional video to play for the participant following the baseline visit. During the viewing, allow the participant to have access to the device and device kit and to follow along at each step.
- Prepare the video to first introduce the participant to the materials and then transition into a step-by-step guide that mimics the flow of an actual study session.
- Present the setup of the tDCS headset slowly and clearly (and make it available for replay), should the user need clarification. Also provide a participant view so that the study participant can gain a sense of what device screens to expect and how the device display will appear when providing certain readings.
- Video sections
Note: Remote technicians will not provide unlock codes for devices unless appropriate contact quality is achieved.- Design the instructional video to detail the means of troubleshooting poor contact quality, including applying pressure and moistening the sponge pockets. Ensure that the video also provides information on the safe usage of study materials, as well as the protocol to follow in order to end a session.
- Instruct participants on procedures to terminate a session and require participants to maintain phone access throughout the duration of the session, ensuring that a level of in-clinic safety is maintained in the case of emergency,
- Following the video, allow the participant to attempt their own set up with the aid of a study technician.
- Instruction manual
- Prepare a transcript of the information presented in the video, as well as photographs and diagrams of the materials, device screenshots, and laptop information, in the form of a manual.
- In-person training
- Monitor the participant as they begin the set-up.
- Coach the participant through each step (moisten sponge pockets, insert electrode into the sponge, fasten sponges on to the headset, place headset on head, center the headset, confirm with study technician, and turn device on).
- Clarify the procedure or equipment where necessary.
- Troubleshooting for participants that experience setup difficulty
- If participants struggle with the set-up, repeat the demonstration of the method.
- If participants are unable to complete the set up after repeated attempts, terminate the session. This is defined as a study "stop" criteria.
5. Participant Preparation for Study Session
- Instruct participants to set up their study laptop, mobile phone, device kit, device and headset in a clean, well lit area. Inform participants to pull hair back and minimize distractions.
- Allow participants to refer to the instructional video or manual for a step by step guide of the protocol.
- First initiate web conference with the study participant before beginning setup so that there is ongoing monitoring for compliance16.
6. Device Setup during Study Session
- After the video conference is initiated, instruct the participant to begin set up.
- Open the sponge pockets and moisten using the 6 mL syringes (one syringe per sponge pocket). About 3 mL can be used to moisten each side of the sponge pocket.
- Apply carbon-rubber electrodes to carry current from the tDCS stimulator. Encase the electrodes in sponge pockets. Insert each carbon rubber electrode into the sponge pocket fully. Note: Since direct skin contact with rubber electrodes is painful, electrodes are encased in sponge pockets.
- Ensure the red cable is connected to red receiver plug labeled 'anode' on the device. Ensure the black cable is connected to black receiver plug labeled 'cathode' on the device. These cables are never removed from the device.
- Connect the sponge pockets and electrodes to the headset using the common bilateral dorsolateral prefrontal cortex (DLPC), with the anodal electrode (one connected to the red wire) placed on the left side. Note: Label the headset with red and black to ensure proper electrode placement.
- Fasten the headset using buttons on the sponge pockets that fit into grooves on the headset. The smooth side of the sponge pocket always faces in toward the forehead.
Note: This offers ease of reliable electrode placement and wide therapeutic applications. - After the sponge pockets are fastened, position the headset on the head. Tuck the back strap beneath the groove in the back of the head and align the center button with the nasion. This can be confirmed using the handheld mirror supplied in the device kit and by the study technician through video conferencing software.
- At this point, instruct the participant to read aloud the contact quality on the device display. Note: A moderate or optimal reading is required for session code administration.
- If contact quality needs to be improved, instruct participants to follow pre-set troubleshooting procedures. First, recommend applying pressure to the sponge pockets. If this does not prove successful, give participants the option to apply a small amount of saline solution to the sponge pockets using the wash bottle (paper towels are provided for any spillage).
- Once proper contact quality is achieved, administer a one-time use unlock code for the sessions. Note: These codes are pre-programmed by study staff for the set amount of time and amplitude.
7. Session completion
Note: After the participant enters the unlock code, the screen of the device will show a timer that counts down the minutes until the end of the session. The device will also indicate the contact quality of the electrodes throughout the session. When 1 min remains on the timer, a countdown in seconds will occur.
- If contact quality falls below "moderate" provide instructions on how to improve it using the troubleshooting steps in 6.9.
- Remind the participant to not to remove the headset until the seconds have fully counted down to zero and the device shuts off (confirmed with a beeping noise).
- Instruct the participant to remove the headset when the device beeps.
- Direct the participant to remove sponge pockets from the device and discard them. Syringes are one time use as well and should also be discarded.
- Ensure that the participant stores the device and headset in the kit.
- Remind the participant to safely store the laptop, device, and all materials for the next session.
8. End of Study Analyses
- Assess each study participant using a participant tracker. This serves as a method of recording study compliance, adverse events, and session completion. At the end of study, review each participant's data to determine study success. For the purpose of this protocol, study success will be defined by 80% of participants having completed 80% of study sessions24.
- Validate session success through records of daily session attendance, review of daily pain scales and before and after stimulation questionnaires for adverse events Please see Supplemental Code Files for sample questionnaire.
Note: Validation that the 20 min of stimulation were fully delivered can be reviewed at study end. The study technician can access the device for completion codes as confirmation.
Representative Results
We have adapted this protocol for use in MS. We targeted the delivery of ten tDCS stimulation sessions delivered over two weeks. 9,10 The first two sessions of the ten were in-person training sessions and the following eight were remotely supervised (Figure 3). The second session consists of an environmental suitability assessment where study technicians visited the participant's home to confirm appropriate set-up.
To complete the following remotely-supervised sessions, participants were provided with the tDCS device specially-designed for remote use and a headset that was modified for ease of use to guide accurate electrode placement. A device kit was provided and included the device and headset, one-time use sponge pockets for electrodes and syringes filled with the measured amount of saline required for each sponge, with all items individually labeled by day and organized for ease of use. Electrodes were placed in the bilateral dorsolateral prefrontal cortex (DLPC) position with the anodal electrode placed on the left side. 10 This offers ease of reliable electrode placement, wide therapeutic applications.9,10 Based on prior studies we targeted 1.5 mA for 20 min sessions. The protocol 9,10allowed for a current reduction to 1.0 mA at baseline if this improves overall subject tolerability.
Participants were given a study-provided laptop computer configured for the study, including the easily accessible instructional video and link for secure video conference connection with the study technician. The laptop also included a program for remote monitoring of all computer activity, and a program to remotely access the computer for technical support. Detailed manuals for operation were used by both the participant and study technician, and a binder for self-report measures was provided.
A total of n=20 MS participants have completed the study. Inclusion criteria specified an Expanded Disability Status Scale (EDSS)25 * of 6.0 or below OR 6.5 or above with proxy to ensure minimal motor requirements to operate the device. Enrollment has been representative of a range of impairment in MS (motor impairment, cognitive impairment, or both). All 20 participants, n=4 with proxy, were successfully trained to self-apply a tDCS session and 192 total sessions were completed. As shown in Figure 4, 40 of the192 sessions included training; the remaining 152 were exclusively remotely-supervised sessions. Of the remotely-supervised sessions, 100% were executed correctly with successfully placement of electrodes, device operation and well-tolerated delivery of stimulation.
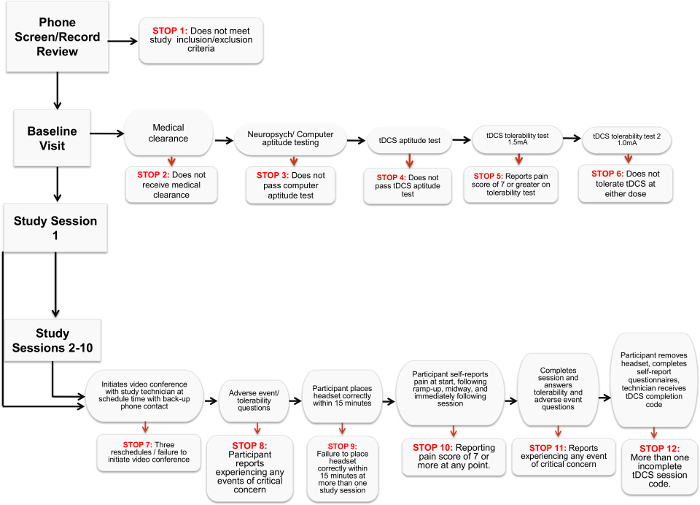
Figure 1. Stop Criteria Flowchart. The chart details the various criteria that indicate a participant can no longer proceed or participate in a remotely-supervised tDCS study. Please click here to view a larger version of this figure.
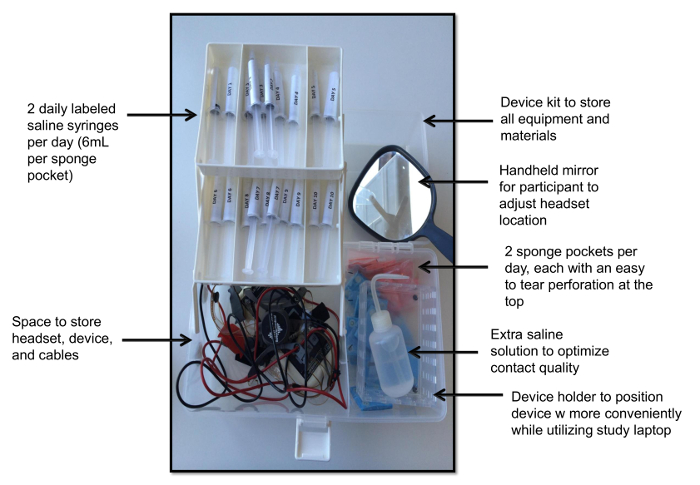
Figure 2. Device Kit. This view demonstrates the device kit with individually wrapped sponge pockets, one saline-filled syringe per sponge per day, a handheld mirror a device holder, spare saline solution, and the device with headgear. Please click here to view a larger version of this figure.
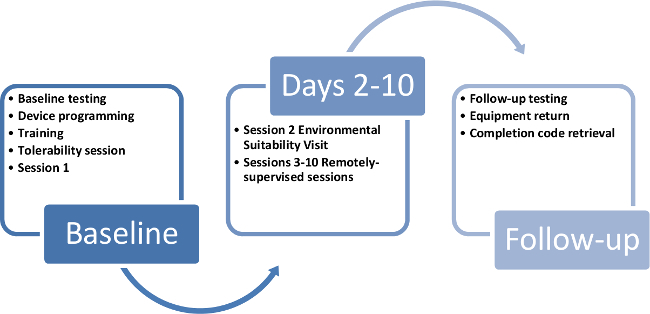
Figure 3. Participant study timeline. This timeline demonstrates a way to cycle study kits and devices through each 10 day participant enrollment in the study. Please click here to view a larger version of this figure.
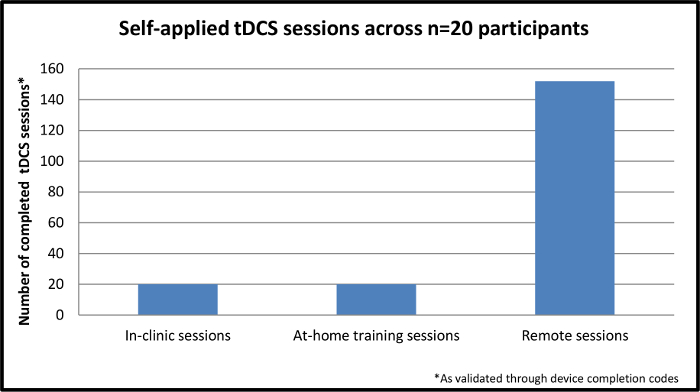
Figure 4. Self-applied tDCS sessions across n=20 participants. This figure demonstrates the completed, self-applied sessions across n=20 participants enrolled in the study. The initial session is completed in-clinic while the remaining nine sessions are completed through remote supervision in the participant's home. Please click here to view a larger version of this figure.
Discussion
Critical steps within the protocol
As remotely-supervised tDCS is administered away from the direct supervision of a clinician, inclusion and exclusion criteria are designed to ensure that the participant has no contraindicated health conditions or environmental distractions, and are fully capable to use a laptop computer (including those with adaptive technology) for communication with the research team. In addition, participants must be able to tolerate a tDCS session and commit to the scheduled session time for the duration of the study.
While remote tDCS offers a convenience to the study and administration of the therapy, self-directed participant use is not advisable due to both safety concerns and the inability to monitor and standardize the stimulation that is delivered. Instead, our protocol follows the standards and guidelines for remotely-supervised tDCS16 to extend clinic standards through delivery in a remote location. The guidelines ensure that research staff are properly trained for participant interactions, that users have proper ability to participate in remote tDCS, and that there are ongoing training materials as well as assessments made of the participant at each step of the study. The stimulation was uniform and reproducible with exactly 20 min of 1.5 mA delivered at every session without any interruption or variation across sessions or individuals.
Modifications to the protocol and troubleshooting tips
The protocol includes several small modifications. Firstly, we expanded the use of this protocol to MS participants that have an EDSS score above 6.5 in the instance where there is a proxy accessible to administer each dose. In addition, we have implemented a procedure whereby we remotely access the participant's study provided laptop to initiate the web conference for those who need extra support and review measures of tolerability and study experiences through a shared document. A future modification to the current protocol includes allowing various degrees of remote supervision so that participants who prove most competent with the technique would only require early supervision to confirm device set up and to receive the unlock code.
Limitations of the technique
While our preliminary results support the feasibility of this protocol, the sample size is limited. As enrollment expands, analyses will be made for gaps in training, ways to streamline sessions, enhance the instructional video, and make the technique more accessible to those with motor impairment (i.e., adaptive mice for computer usage, sponge pocket/headset modification to further ease application). Some participants in the EDSS range below 6.5 (not requiring proxy), may still experience some difficulty in headset preparation and troubleshooting computer related issues. Furthermore, while this study recommends full remote monitoring of participants throughout all sessions, future studies may deem some participants sufficiently trained to operate the device without supervision for the entirety of a session.
Significance of the method with respect to existing methods
These initial results demonstrate the feasibility of our protocol for remotely-supervised tDCS delivery for clinical trials, following a set of guidelines and standards that must be employed to safely, and effectively administer tDCS under remote supervision. The protocol was designed to have a decision-tree series of checkpoints with "stop" criteria (section 2.5.1 above) that must be cleared in order to proceed at each step (see Figure 1). These checkpoints addressed tolerability (experiences of pain or adverse effects to the treatment) and compliance (timely session attendance and proper technique). For each session 1 through 10, participants completed brief adverse event reports before and after their sessions (with items derived from a list of the most common tDCS side-effects in previous trials). In addition, participants completed the self-report measures to address tolerability (before and after the session) and can complete symptom inventories as well. This study is significant in that it establishes a technique to examine a therapy in MS with adequate power while also providing broader access to tDCS treatment.
Future applications of the technique
Once the method for remotely-supervised tDCS has been fully piloted in the MS population, a larger, randomized controlled trial can be initiated to target symptom management. Through the use of the instructional training materials and structure around daily participant interactions, remotely-supervised tDCS can be accessed by a wider range of patient populations and expand clinical study of the technique.
開示
The authors have nothing to disclose.
Acknowledgements
Supported by The Lourie Foundation, Inc.
Materials
| Mini-CT transcranial direct current stimulation device | Soterix | Device to deliver direct current stimultion in a remote manner | |
| Study Kit | NA | Provided to participant with all required setup items – device, headset, sponge pockets, pre-filled syringes, Kleenex, handheld mirro, spare batteries | |
| Laptop | NA | Provided to allow secure video conferecing during device setup and headset placement | |
| Instruction Manual | NA | Transcription of instructional video and detailed instructions for protocol |
参考文献
- Filmer, H. L., Dux, P. E., Mattingley, J. B. Applications of transcranial direct current stimulation for understanding brain function. Trends in neurosciences. 37, 742-753 (2014).
- Brunoni, A. R., et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation. 5, 175-195 (2012).
- Nitsche, M. A., et al. Transcranial direct current stimulation: State of the art 2008. Brain stimulation. 1, 206-223 (2008).
- Iyer, M. B., et al. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 64, 872-875 (2005).
- Vanneste, S., et al. Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Experimental brain research. 202, 779-785 (2010).
- Priori, A., Hallett, M., Rothwell, J. C. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation?. Brain stimulation. 2, 241-245 (2009).
- Demirtas-Tatlidede, A., Vahabzadeh-Hagh, A. M., Pascual-Leone, A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders. Neuropharmacology. 64, 566-578 (2013).
- Martin, D. M., et al. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 16, 1927-1936 (2013).
- Elmasry, J., Loo, C., Martin, D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restorative neurology and neuroscience. 18 (6), 065018 (2015).
- Brunoni, A. R., Vanderhasselt, M. A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain and cognition. 86, 1-9 (2014).
- Meesen, R. L., Thijs, H., Leenus, D. J., Cuypers, K. A single session of 1 mA anodal tDCS-supported motor training does not improve motor performance in patients with multiple sclerosis. Restorative neurology and neuroscience. 32, 293-300 (2014).
- Shiozawa, P., et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 17, 1443-1452 (2014).
- Loo, C. K., et al. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. The British journal of psychiatry : the journal of mental science. 200, 52-59 (2012).
- Lampit, A., Hallock, H., Valenzuela, M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS medicine. 11, e1001756 (2014).
- Magalhaes, R., et al. Are cognitive interventions for multiple sclerosis effective and feasible. Restorative neurology and neuroscience. 32, 623-638 (2014).
- Charvet, L. E., et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Frontiers in systems neuroscience. 9, 26 (2015).
- Palm, U., Ayache, S. S., Padberg, F., Lefaucheur, J. P. Non-invasive Brain Stimulation Therapy in Multiple Sclerosis: A Review of tDCS, rTMS and ECT Results. Brain stimulation. 7, 849-854 (2014).
- Mori, F., et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. The journal of pain : official journal of the American Pain Society. 11, 436-442 (2010).
- Mori, F., et al. Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain stimulation. 6, 654-659 (2013).
- Tecchio, F., et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. Journal of neurology. 261, 1552-1558 (2014).
- Saiote, C., et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restorative neurology and neuroscience. 32, 423-436 (2014).
- Ferrucci, R., et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 34, 121-127 (2014).
- Cuypers, K., et al. Anodal tDCS increases corticospinal output and projection strength in multiple sclerosis. Neuroscience letters. 554, 151-155 (2013).
- Zanao, T. A., et al. Impact of two or less missing treatment sessions on tDCS clinical efficacy: results from a factorial, randomized, controlled trial in major depression. Neuromodulation : journal of the International Neuromodulation Society. 17, 737-742 (2014).
- Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 33, 1444-1452 (1983).

