Visible-light Induced Reduction of Graphene Oxide Using Plasmonic Nanoparticle
概要
A simple protocol for the preparation of reduced graphene oxide using visible light and plasmonic nanoparticle is described.
Abstract
Present work demonstrates the simple, chemical free, fast, and energy efficient method to produce reduced graphene oxide (r-GO) solution at RT using visible light irradiation with plasmonic nanoparticles. The plasmonic nanoparticle is used to improve the reduction efficiency of GO. It only takes 30 min at RT by illuminating the solutions with Xe-lamp, the r-GO solutions can be obtained by completely removing gold nanoparticles through simple centrifugation step. The spherical gold nanoparticles (AuNPs) as compared to the other nanostructures is the most suitable plasmonic nanostructure for r-GO preparation. The reduced graphene oxide prepared using visible light and AuNPs was equally qualitative as chemically reduced graphene oxide, which was supported by various analytical techniques such as UV-Vis spectroscopy, Raman spectroscopy, powder XRD and XPS. The reduced graphene oxide prepared with visible light shows excellent quenching properties over the fluorescent molecules modified on ssDNA and excellent fluorescence recovery for target DNA detection. The r-GO prepared by recycled AuNPs is found to be of same quality with that of chemically reduced r-GO. The use of visible light with plasmonic nanoparticle demonstrates the good alternative method for r-GO synthesis.
Introduction
The first developed scotch-tape based method1 and chemical vapor deposition2 were excellent methods to produce the pristine state of a graphene, but the large scale graphene synthesis or graphene layer formation on the surface with wide area have been regarded as a key limitation of previous methods.3 One of possible solution for large scale r-GO synthesis will be wet-chemical synthetic method which first requires the reactions with strong oxidants, extensive physical treatment such as sonication to produce GO sheet, and finally the reduction of oxygen functionalities such as hydroxy, epoxide and carbonyl groups in GO is essential in order to recover its original physical properties.4 Mostly, the reduction of GO was carried out with either chemical method using hydrazine or its derivatives5 or by thermal treatment method (550–1,100 °C) in an inert or reducing atmosphere.6
These processes require the toxic chemicals, long reaction time and high temperature which increased total energy demand for r-GO synthesis.7 While the photo-irradiating reduction processes such as UV-induced,8 photo-thermal process using a pulsed xenon flash,9 pulsed laser assisted10 and photo-thermal heating with camera flash lights11 have also been reported for the preparation of r-GO. In general, the low conversion efficiency of the photo-induced methods propagated to the use of UV or pulsed laser irradiation that can deliver high photon energy. The low photon energy of visible light limits its use and not attracted much for r-GO synthesis. Excellent light absorption properties of plasmonic nanoparticles in the visible and/or NIR regions can greatly improve the current drawbacks of the use of visible light for r-GO synthesis.12,13 Mild reaction conditions, short reaction time and limited use of toxic chemicals could make the visible light induced plasmon assisted photocatalytic reduction of GO as a useful alternative method.
In present method, we describe the efficient and simple r-GO synthetic method using plasmonic nanoparticles and visible light. The reaction progress was found to be strongly dependent on the structures of plasmonic nanoparticles such as spherical gold nanoparticles (AuNPs), gold nanorods (AuNRs), and gold nanostars (AuNSs). The use of AuNPs showed the most efficient reduction of GO and the nanoparticles are easily removable and recyclable for the repeated use (Figure 1). The r-GO synthesized using visible light and AuNPs showed almost equal quality compared with the r-GO prepared by well-known chemical method (hydrazine) as demonstrated by use of various analytical measurements and the fluorescence quenching/recovery based DNA detection method.
Protocol
1. Preparation of Precursor
- Preparation of graphene oxide (GO):
- GO preparation using modified Hummer’s method14
- Add 3.0 g of graphite flakes to a mixture of concentrated H2SO4/H3PO4 (360:40 ml) at RT. (Note: Special care has to be taken while using strong acids H2SO4 and H3PO4.)
- Add KMnO4 (18.0 g) slowly with stirring and cooling in an ice bath to maintain the temperature of reaction mixture at < 35 °C. (The solution become sticky with increased reaction time, need to use proper method to maintain efficient stirring.) (Note: Special care has to be taken while adding KMnO4 due to exothermic reaction.)
- Stir for 12 hr at 50 °C and then cool to RT and pouring reaction mixture onto ice (400 ml) containing 30% H2O2 (3 ml).
- Filter the reaction mixture using a metal U.S. Standard testing sieve (300 µm) to remove unreacted graphite and centrifuge the filtrate (4,722 x g speed for 2 hr) to remove the supernatant.
- Repeat the centrifugation step with 200 ml of water, 200 ml of 30% HCl, 200 ml of ethanol, and distilled water again until pH of solution reach at 5.0–6.0.
- Lyophilize the final solutions to produce a fluffy GO powder.
- In order to make nanosized GO solution, dissolve 20 mg of GO powder in 40 ml of triple distilled water (> 18 MΩ), and then exfoliate by prolonged sonication (35% amplitude, 500 W, 2 hr) until the entire size distribution become below 150 nm, then centrifuge it two times (10,625 x g speed, 15 min) to remove precipitates (un-exfoliated large GO sheets).
- GO preparation using modified Hummer’s method14
- Preparation of plasmonic nanoparticle
- Preparation of AuNPs
- Citrate-stabilized spherical shape gold nanoparticle (AuNPs, O.D. = 1.0) of 30 nm particles size was used for r-GO reduction.
- Preparation of AuNRs15
- Prepare the seed solution by adding a freshly prepared 0.6 ml ice-cold solution of NaBH4 solution (0.01 M) into an aqueous mixture solution compose of 0.25 ml of HAuCl4 (0.01 M) and 9.75 ml of cetyltrimethylammonium bromide (CTAB, 0.1 M).
- Stir the resulting mixture vigorously for 0.5 min and then keep it at 28 °C for 3 hr.
- Prepare the growth solution by mixing 475 ml of CTAB (0.1 M), 3 ml of AgNO3 (0.01 M) and 20 ml of HAuCl4 (0.01 M).
- Then add freshly prepared 3.2 ml of ascorbic acid (0.01 M) to the mixture followed by the addition of 0.8 ml of an aqueous HCl (1.0 M) solution.
- In the final step add 3.2 ml of seed solution to the growth solution at 28 °C and subject the reaction mixture to quick inversion for few seconds. Finally, keep the resulting mixture undisturbed for at least 6 hr.
- Analyze the prepared AuNRs with UV-Visible spectroscopy for absorption maxima (λmax) and TEM analysis (typically the λmax and aspect ratio was found to be 730 nm and 3.5, respectively).
- Preparation of AuNSs16
- Prepare aqueous stock solution of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) with concentration of 100 mM, and adjust the pH to 7.4 at 25 °C by adding 1.0 M NaOH solution.
- Mix 20 ml of phosphate buffer (100 mM) with 30 ml of 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (100 mM).
- Then add 500 µl of gold(III) chloride trihydrate (20 mM) to the above mixture and keep at 28.5 °C for 30 min in water bath. Solution color changes from light yellow to greenish blue after 30 min could be observed.
- Centrifuge the solution at 8,928 x g speed for 30 min and disperse the precipitates in distilled water.
- Finally, analyze the prepared AuNSs with UV-Visible spectroscopy for absorption maxima (λmax) and TEM analysis for particles size confirmation which is found to be 740 nm and 30 nm, respectively.
- Preparation of AuNPs
2. Preparation of r-GO Using Visible Light and AuNPs
- Add 1 ml of plasmonic nanoparticles (Abs 1.0 at 520 nm for AuNPs, Abs 1.0 at 750 nm for AuNRs, and Abs 1.0 at 730 nm for AuNSs, respectively) and 100 µl of ammonium hydroxide (28%, w/v%) to 10 ml of GO solution (OD 1.0 at 230 nm, 0.125 mg ml-1) placed in a Pyrex glass reactor equipped with a water-circulating jacket.
- Irradiate the mixture with Xe lamp (power density of 1.56 W cm-2) for 30 min with water circulation through water-circulating jacket to maintain the temperature at 25 °C and then centrifuge the solution at 10,625 x g speed for 15 min to remove gold nanoparticles.
- Take the supernatant containing the prepared r-GO to analyze with UV-Visible spectrophotometer (r-GO should show the distinctive absorption band at 270 nm) in the range of 200–900 nm.
3. Target DNA Detection Using r-GO Solution17
- For fluorescence quenching, add 20 µl of 10-6 M Cy3-modified ssDNA (5'-ATC CTT ATC AAT ATT TAA CAA TAA TCC CTC-Cy3-3') into GO or r-GO solution containing 25 µl of GO (0.125 mg ml-1) or r-GO (0.125 mg ml-1) in 1,955 µl of 0.3 M PBS solution (10 mM phosphate buffer, 0.3 M NaCl) and incubate for 10 min at RT.
- Measure the fluorescence intensity of these samples with spectrofluorometer (λex= 529 nm).
- For Target Detection, add 200 µl of target oligonucleotide solution (5'- GAG GGA TTA TTG TTA AAT ATT GAT AAG GAT- 3') in three different concentrations (10-6 M, 10-7 M, 10-8 M) into the GO or r-GO solution containing 20 µl of 10-6 M ssDNA-Cy3, 25 µl of GO or r-GO (0.125 mg ml-1), and 1,755 µl of 0.3 M PBS for fluorescence recovery experiment.17
Notes:
Light Sources & Reactor
Visible light (400–780 nm) source. Visible light irradiate through Pyrex glass reactor (window diameter = 1.1 cm) containing GO solution using Xe lamp (1.56 W/cm2 power). The photon energy applied to the reactor is calculated to be 4.8 × 1021 photons per min (Figure 2A-2C).
Near-infrared (NIR) laser. NIR laser (window diameter = 13.2 cm) with power density of 0.36 W/cm2, and operating wavelength of 808 nm has been used as the source of near-infrared light for GO reduction reactions (Figure 2E). The photon energy is calculated to be 2.43 × 1021 photons per min.
Reactor: Pyrex glass reactor (window diameter = 1.1 cm; reaction volume = 10 ml) equipped with a water-circulating jacket is used for both visible light and NIR light irradiated GO reduction reactions (Figure 2F).
Representative Results
Figure 1 shows the overall scheme for visible light and plasmonic nanoparticle based r-GO reduction reaction. Figure 2 shows the instrumental setup for the reactions. After reaction, it is required the centrifugation step to remove the used photocatalyst (AuNSs, AuNRs, or AuNPs) as shown in Figure 3A. The HRTEM analysis shows the complete removal of nanoparticles in the supernatant (r-GO) (Figure 3B), which is also possible to confirm with UV-Visible analysis as shown in Figure 3C, the absorption band around 500–800 nm from the r-GO and nanoparticle mixture solution was disappeared after centrifugation step indicating the complete removal of plasmonic nanoparticles in r-GO product. The structural changes in the r-GO have been analyzed by XRD technique. Disappearance of GO peak at 10.2 clearly indicated the formation of r-GO as shown in Figure 4A. The D/G intensity ratios (ID/IG) of GO and r-GO produced by a chemical method or a light-induced method without or with NPs (AuNRs, AuNPs, and AuNSs) was measured by Raman analysis as shown in Figure 4B. The formation of r-GO was confirmed more quantitatively by comparing the C/O ratios in XPS analysis between samples as shown in Figure 5. By dividing area % of carbon (C) with the area % of oxygen (O), the C/O ratios of the prepared r-GO could be calculated, the higher number of C/O ratio indicate the higher degree of reduced state of r-GO. As shown in Figure 5, the C/O ratio of GO, r-GO (chemically reduced with hydrazine), r-GO (visible light only), and r-GO (visible light and plasmonic nanoparticle) were 1.95, 4.81, 3.74, and 5.19. These results show the usefulness of visible light and plasmonic nanoparticle based method for the preparations of r-GO.
The fluorescence quenching efficiency and recovery for target DNA detection have been performed to demonstrate the potentials of r-GO for bio applications. Figure 6A is the summarized fluorescence emission spectra of Cy3-modified DNA after incubating with GO, r-GO solutions in 0.3 M PBS, the decreased intensity indicates the efficient quenching efficiency of GO, r-GO. The r-GO prepared with AuNPs and visible light showed the most efficient quenching efficiency. When the Cy3-modified DNA bound with target DNA (anthrax DNA in this paper), the Cy3-modified DNA could form duplex and separated from the r-GO sheet, which result in the fluorescence recovery (Figure 6B). It is believed that the prepared r-GO using visible light and plasmonic nanoparticle shows as excellent as physical properties of chemically reduced r-GO (Figure 6).
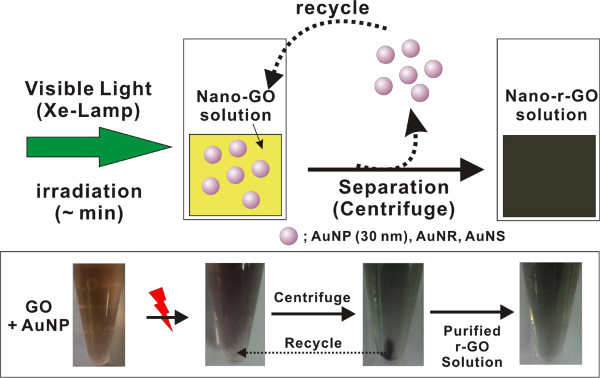
Figure 1. Reaction scheme for r-GO synthesis. Schematic description of r-GO synthesis using plasmonic nanoparticle and visible light. (Re-print with the permission from ref. 13) Please click here to view a larger version of this figure.
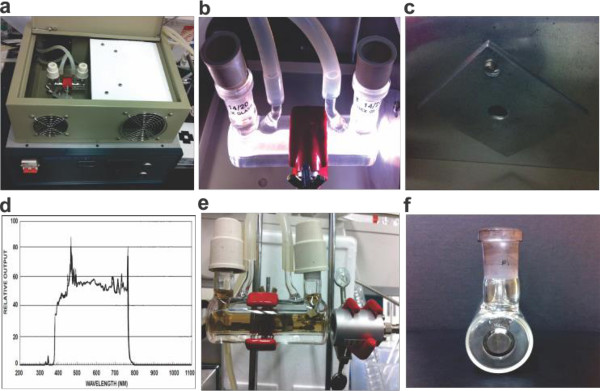
Figure 2. The reactor and light sources for r-GO preparation. The photographs of (A) reactor with water circulation jacket in the box equipped with visible light source (Xe lamp), (B) enlarged images of reactor, (C) the hole to guide visible light into the reactor, (D) the spectrum of visible light from Xe-lamp, (E) NIR laser with reaction apparatus, (F) the side view of reactor (Pyrex, window diameter = 11 mm). Please click here to view a larger version of this figure.
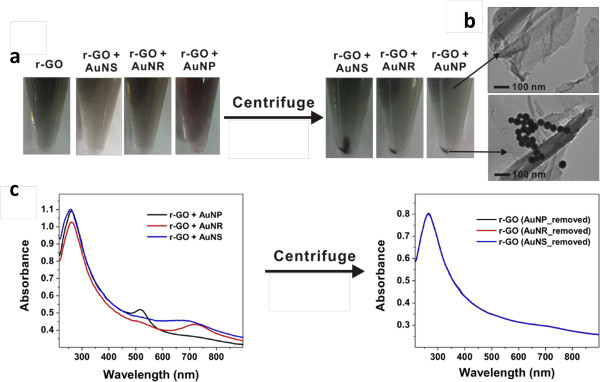
Figure 3. Photographs, HR-TEM images and UV-Vis spectrum of r-GO. (A) The photographs of r-GO, r-GO + AuNPs, r-GO + AuNRs, r-GO + AuNSs solution before and after centrifugation, (B) HR-TEM images of r-GO solution and precipitates, (C) the UV-Vis spectra of r-GO + AuNPs mixture, r-GO + AuNRs mixture, and r-GO + AuNSs mixture before and after centrifugation. (Re-print with the permission from ref. 13) Please click here to view a larger version of this figure.
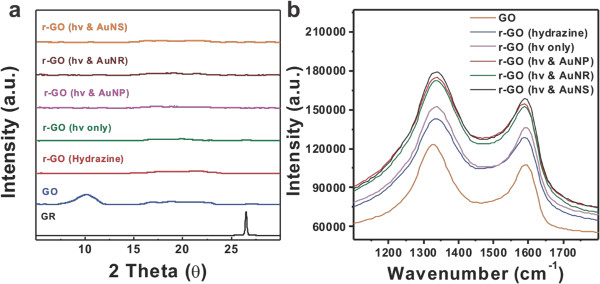
Figure 4. Qualitative analysis of GO and r-GO prepared. (A) XRD data; (B) Raman spectra of GO and r-GO produced by a chemical method and a light-induced method with or without AuNPs, AuNRs, and AuNSs. (Re-print with the permission from ref. 13) Please click here to view a larger version of this figure.
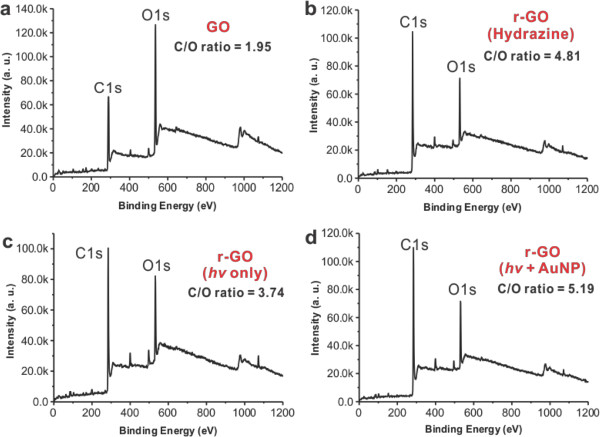
Figure 5. The XPS analysis of r-GO. The XPS analysis of GO solution (A), r-GO solutions prepared with chemical method (B), and light-induced method without AuNPs (C) or with AuNPs (D). (Re-print with the permission from ref. 13) Please click here to view a larger version of this figure.
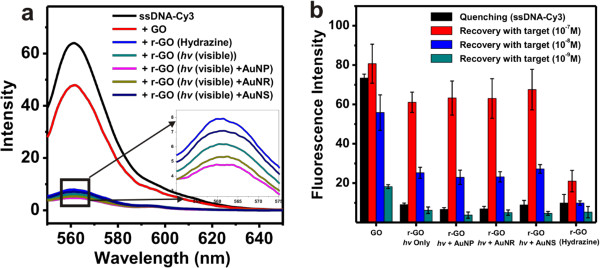
Figure 6. Fluorescence quenching and fluorescence recovery analysis. (A) Fluorescence quenching of ssDNA-Cy3 using GO and r-GO chemically reduced or produced using visible light and plasmonic nanoparticles, (B) fluorescence recovery using varying concentrations of target DNA (10-7 M, 10-8 M and 10-9 M). Data are means ± standard deviations, N=4. (Re-print with the permission from ref. 13) Please click here to view a larger version of this figure.
Discussion
Visible light irradiation onto GO solution for 30 min with gold nanoparticles (AuNPs, AuNSs & AuNRs) showed the rapid color changes from light yellow-brown to black color (Figure 1). To obtain highly pure r-GO product in high yield, there are two important factors need to follow. One is the use of AuNPs as an efficient plasmonic catalyst, since AuNPs can strongly absorb the visible light among other structures (i.e., AuNRs, AuNSs). Another is the use of nanosized GO solution to obtain nanoparticle-free highly pure r-GO product. The used plasmonic nanoparticles as a photocatalyst should be completely removed, which is easily achievable by applying simple centrifugation steps at 10,625 x g speed for 15 min. But in doing this, the large sheet size of r-GO (> 500 nm) can be centrifuged down with gold nanoparticles which lead to the great loss of the product (r-GO). Therefore, using nanosized GO solution (average sheet size < 150–200 nm) is very important, because the nanosized r-GO is not possible to be centrifuged down using such a usual centrifugation conditions (i.e., 10,625 x g speed for 15 min).
Therefore, the use of AuNPs and the use of nanosized GO solution are critical factors to obtain highly pure r-GO solution in a high yield. To get powder form of product, it is required to perform additional freeze drying steps. To confirm the successful formation of r-GO, the measurement of UV spectra will be one of simple method. The red shift in UV-Vis spectra from 230 nm to 270 nm is one of clear indication of the successful conversion of GO into r-GO (Figure 3C). To verify the complete removal of used gold nanoparticles, the measurement with UV-Vis and HR-TEM analysis are also required as shown in Figure 3B & 3C.
The disappearance of graphite peak at 26.48 in GO and the (001) peak at 10.2 corresponding to GO in the XRD spectrum showed the successful formation of r-GO (Figure 4A). GO and r-GO prepared by a chemical method or a light-induced method were analyzed qualitatively by Raman spectrometry as displayed in Figure 4B. The D band corresponding to disordered structures and edge planes and the G band corresponding to ordered sp2 bonded carbon appeared at 1,327 cm-1 and 1,590 cm-1, respectively, in the Raman spectrum of GO.18 The D and G bands at 1,336 and 1,592 cm-1 were also present in the Raman spectrum of the chemically reduced GO, visible light irradiated reduced GO and plasmon assisted visible light irradiated reduction of GO. The D/G intensity ratios (ID/IG) were found to be 1.03, 1.13, 1.12, 1.13, 1.13 and 1.13 for GO and r-GO prepared by a chemical method or a light-induced method without or with NPs (AuNRs, AuNPs, and AuNSs), respectively. The XPS analysis is the most convincing and quantitative analytical method to verify the successful conversion of GO into r-GO product. The C/O ratios (based on the intensity of each element (carbon and oxygen) were found to be 1.95, 4.81, 3.74, and 5.19 for GO, r-GO (hydrazine), r-GO (hv only), and r-GO (hv + AuNPs), respectively (Figure 5).
The possible limitation of the current method for r-GO synthesis is the required light source such as Xe-lamp for reactions. But one possible, promising and ultimate solution for this limitation is the use of sun light as a light source since the sun light are mainly composed of UV and visible light. But in this case, longer illumination time is expected to be the one possible problem.
There are numerous potential applications of r-GO,19-24 one of important properties for bio application is the fluorescence quenching effect of r-GO. In this protocol, we described simple application of using r-GO for sensitive DNA detection scheme. As described the results in Figure 6, the r-GO prepared using visible light and plasmonic nanoparticles showed excellent properties for fluorescence quenching and recovery in the presence of the target DNA as compared to r-GO prepared by chemical method (Figure 6B).
In this protocol, we described the simple synthetic method for r-GO using visible light and its analytical method and applications. As discussed, the future modifications of this method will be the use of sun light which regarded as the most eco-friendly energy sources for reactions.
開示
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Research Foundation of Korea (2013R1A1A1061387) and KU-KIST research fund.
Materials
| Cy3 modifeid ssDNA | IDT(Iowa, USA) | HPLC purified by IDT | |
| Gold nanoparticles (30 nm) | Ted Pella, Inc(Redding, CA, USA). | 15706-20 | colloidal solution |
| 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) (99.5%) | Sigma-Aldrich (St. Louis, MO, USA) | 7365-45-9 | |
| Gold(III)Chloride Hydrate (99.999%) | Sigma-Aldrich (St. Louis, MO, USA) | 27988-77-8 | strongly hygroscopic |
| Sodium Borohydride (99.99%) | Sigma-Aldrich (St. Louis, MO, USA) | 16940-66-2 | |
| Hexadecyltrimethylammonium bromide (≥99%) | Sigma-Aldrich (St. Louis, MO, USA) | 57-09-0 | |
| L-Ascorbic Acid(≥99.0%) | Sigma-Aldrich (St. Louis, MO, USA) | 50-81-7 | |
| Sodium Chloride (99.5%) | Sigma-Aldrich (St. Louis, MO, USA) | 7647-14-5 | |
| Silver Nitrate (≥99.0%) | Sigma-Aldrich (St. Louis, MO, USA) | 7761-88-8 | |
| Graphite | Sigma-Aldrich (St. Louis, MO, USA) | 7782-42-5 | |
| Sulfuric acid | Sigma-Aldrich (St. Louis, MO, USA) | 7664-93-9 | |
| Phophoric acid | Sigma-Aldrich (St. Louis, MO, USA) | 7664-38-2 | |
| Potassium permanganate | Sigma-Aldrich (St. Louis, MO, USA) | 7722-64-7 | |
| Hydrogen peroxide | JUNSEI | 23150-0350 | |
| Ammonium hydroxide | Sigma-Aldrich (St. Louis, MO, USA) | 1336-21-6 | |
| Xe-lamp | Cermax, Waltham, USA | ||
| NIR Laser | Class-IV, Sanctity Laser, Shanghai, China | 6W (output power) | |
| UV-Vis spectrophotometer | S-3100, SINCO, South Korea | ||
| Transmission Electron Microscopy | H-7650, Hitachi, Japan | ||
| Spectro Fluorometer | Jasco FP-6500, Tokyo, Japan | ||
| X-ray Photoelectron Spectrometer | AXIS–NOVA, KRATOS Inc., UK |
参考文献
- Novoselov, K. S., et al. Electric Field Effect in Atomically Thin Carbon Films. Science. 306 (5696), 666-669 (2004).
- Obraztsov, A. N. Chemical Vapour Deposition Making Graphene on a Large Scale. Nat Nanotechnol. 4 (4), 212-213 (2009).
- Choi, W., Lahiri, I., Seelaboyina, R., Kang, Y. S. Synthesis of Graphene and Its Applications A Review. Crit Rev Solid State Mater Sci. 35 (1), 52-71 (2010).
- Zhou, Y., et al. Microstructuring of Graphene Oxide Nanosheets Using Direct Laser Writing. Adv Mater. 22 (1), 67-71 (2010).
- Stankovich, S., et al. Graphene Based Composite Materials. Nature. 442 (7100), 282-286 (2006).
- Becerril, H. A., et al. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano. 2 (3), 463-470 (2008).
- Wang, X., Zhi, L., Müllen, K. Transparent Conductive Graphene Electrodes for Dye Sensitized Solar Cells. Nano Lett. 8 (1), 323-327 (2007).
- Sungjin, P., Ruoff, R. S. Chemical Methods for the Production of Graphenes. Nat Nanotechnol. 4 (4), 217-224 (2009).
- Akhavan, O., Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J Phys Chem C. 113 (47), 20214-20220 (2009).
- Huang, L., et al. Pulsed Laser Assisted Reduction of Graphene Oxide. Carbon. 49 (7), 2431-2436 (2011).
- Cote, L. J., Cruz-Silva, R., Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer. Composite. J. Am. Chem. Soc. 131 (31), 11027-11032 (2009).
- Wu, T., et al. Surface plasmon resonance-induced visible light photocatalytic reduction of graphene oxide: Using Ag nanoparticles as a plasmonic photocatalyst. Nanoscale. 3 (5), 2142-2144 (2011).
- Kumar, D., Kaur, S., Lim, D. K., et al. Plasmon-assisted and visible-light induced graphene oxide reduction and efficient fluorescence quenching. Chem. Commun. 50 (88), 13481-13484 (2014).
- Gilje, S., et al. Photothermal Deoxygenation of Graphene Oxide for Patterning and Distributed Ignition Applications. Adv. Mater. 22 (3), 419-423 (2010).
- Cote, L. J., Kim, F., Huang, J. Langmuir−Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 131 (3), 1043-1049 (2008).
- Ming, T., et al. Experimental Evidence of Plasmophores: Plasmon-Directed Polarized Emission from Gold Nanorod–Fluorophore Hybrid Nanostructures. Nano Lett. 11 (6), 2296-2303 (2011).
- Xie, J., Lee, J. Y., Seedless Wang, D. I. C. Surfactantless, High-Yield Synthesis of Branched Gold Nanocrystals in HEPES Buffer Solution. Chem. Mater. 19 (11), 2823-2830 (2007).
- Ferrari, A. C., Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamond like carbon. Phys. Rev. B. 64 (7), 075414-1-075414-13 (2000).
- Tian, J., et al. One–pot green hydrothermal synthesis of CuO–Cu2O–Cu nanorod–decorated reduced graphene oxide composites and their application in photocurrent generation. Catal. Sci. Technol. 2 (11), 2227-2230 (2012).
- Liu, S., Tian, J., Wang, L., Sun, X. A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. CARBON. 49 (10), 3158-3164 (2011).
- Tian, J., et al. Environmentally Friendly, One–Pot Synthesis of Ag Nanoparticle Decorated Reduced Graphene Oxide Composites and Their Application to Photocurrent Generation. Inorg. Chem. 51 (8), 4742-4746 (2012).
- Tian, J., et al. Three–Dimensional Porous Supramolecular Architecture from Ultrathin gC3N4 Nanosheets and Reduced Graphene Oxide: Solution Self-Assembly Construction and Application as a Highly Efficient Metal–Free Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces. 6 (2), 1011-1017 (2014).
- Liu, S., et al. Stable Aqueous Dispersion of Graphene Nanosheets: Noncovalent Functionalization by a Polymeric Reducing Agent and Their Subsequent Decoration with Ag Nanoparticles for Enzymeless Hydrogen Peroxide Detection. Macromolecules. 43 (23), 10078-10083 (2010).
- Li, H., et al. Photocatalytic synthesis of highly dispersed Pd nanoparticles on reduced graphene oxide and their application in methanol electro–oxidation. Catal. Sci. Technol. 2 (6), 1153-1156 (2012).

