Production of Apolipoprotein C-III Knockout Rabbits using Zinc Finger Nucleases
概要
Recent development in gene targeting tools makes production of knockout (KO) rabbits possible. In the present work, we generated five Apolipoprotein (Apo) C-III KO rabbits using Zinc Finger Nucleases (ZFN). This work demonstrated that ZFN is a highly efficient method to produce KO rabbits.
Abstract
Apolipoprotein (Apo) C-III (ApoCIII) resides on the surface of plasma chylomicron (CM), very low density lipoprotein (VLDL) and high density lipoproteins (HDL). It has been recognized that high levels of plasma ApoCIII constitutea risk factor for cardiovascular diseases (CVD). Elevated plasma ApoCIII level often correlates with insulin resistance, obesity, and hypertriglyceridemia. Invaluable knowledge on the roles of ApoCIIIin lipid metabolisms and CVD has been obtained from transgenic mouse models including ApoCIII knockout (KO) mice; however, it is noted that the metabolism of lipoprotein in mice is different from that of humans in many aspects. It is not known until now whether elevated plasma ApoCIII is directly atherogenic. We worked to develop ApoCIII KO rabbits in the present study based on the hypothesis that rabbits can serve as a reasonablemodelfor studying human lipid metabolism and atherosclerosis. Zinc finger nuclease (ZFN) sets targeting rabbit ApoCIIIgene were subjected to in vitro validation prior to embryo microinjection. The mRNA was injected to the cytoplasm of 35 rabbit pronuclear stage embryos, and evaluated the mutation rates at the blastocyst state. Of sixteen blastocysts that were assayed, a satisfactory 50% mutation rate (8/16) at the targeting site was achieved, supporting the use of Set 1 for in vivo experiments. Next, we microinjected 145 embryos with Set 1 mRNA, and transferred these embryos to 7 recipient rabbits. After 30 days gestation, 21 kits were born, out of which five were confirmed as ApoCIII KO rabbits after PCR sequencing assays. The KO animal rate (#KO kits/total born) was 23.8%. The overall production efficiency is 3.4% (5 kits/145 embryos transferred). The present work demonstrated that ZFN is a highly efficient method to produce KO rabbits. These ApoCIII KO rabbits are novel resources to study the roles of ApoCIII in lipid metabolisms.
Introduction
Apolipoprotein (Apo) C-III (ApoCIII) is a small O-glycosylated secretory protein that is synthesized mainly in the liver and intestine. It resides on the surface of plasma chylomicron (CM), very low density lipoprotein (VLDL) and high density lipoproteins (HDL). ApoCIII has been recognized as a risk factor for cardiovascular disease1. Patients with hereditary deficiency of ApoCIII have low plasma triglyceride (TG) levels and reduced subclinical coronary artery atherosclerosis2,3 . Elevated plasma ApoCIII level, on the other hand, often correlates with insulin resistance, obesity, and hypertriglyceridemia (HTG)4,5 .
Gene knockout (KO) is a powerful means to study the function of a gene. Consistent with the observations in human mutant populations, knockout of the ApoCIII gene in mice led to a reduction of plasma TG and protection from postprandial HTG6. Such positive role of ApoCIII appeared to be independent of Apolipoprotein E (ApoE), as mice deficient of both ApoE and ApoCIII are also protected from postprandial hyperlipidemia7. Although these ApoCIII KO mouse models have provided invaluable information on the possible functions of ApoCIII in humans, it is noted that the metabolism of lipoprotein of mice is different from that of humans in many aspects. For example, mouse lacks plasma cholesteryl ester transfer protein (CETP), a major enzyme involved in the transportation of VLDL, LDL, and HDL8. Therefore, it is necessary to develop an appropriate animal model to further understand the physiological role of ApoCIII in vivo.
The rabbit is a classical model animal species9-11. It has a short gestation period (30-31 days), large litter size (4-12/litter) and can be housed conveniently in an indoor facility. Compared to mice, rabbits are phylogenetically closer to humans10. Importantly, like human but unlike mice, rabbits are LDL-rich mammals and have substantial levels of CETP10,12 . Furthermore, they are susceptible to cholesterol-rich diet-induced atherosclerosis with the lesions similar to those seen in human atherosclerosis13. For these reasons, we hypothesized that ApoCIII KO rabbits can serve as a better model than their mouse counterparts to investigate the roles of ApoCIIIplay in lipid metabolism and atherosclerosis in humans.
Production of gene targeted transgenic (GTT) rabbits has been a challenge. This is mainly due to the lack of germline transmitting embryonic stem cells (ESC), and the extremely low efficiency of somatic cell nuclear transfer (SCNT) in rabbits. ESC is the primary tool to generate GTT mice, whereas and SCNT has been successfully applied to generate KO animals in species lacking germline transmitting ESCs, including pigs, sheep and cattle, but not rabbits. Recently Zinc Finger Nuclease (ZFN), Transcription Activator-Like Effector Nuclease (TALEN)14, and RNA Guided Endonuclease (RGEN)15emerged as powerful means for genome editing. These nucleases, so called "molecular scissors", are efficient in generating double-strand breaks (DSB) in the genome that can lead to a functional KO of the targeted gene or used to integrate a DNA sequence at a specific locus in the genome in a number of species16. In 2011, among the first adapting the ZFN technology, we generated peroxisome proliferator-activated receptor gamma (PPARγ) KO porcine cells via this approach and successfully generated PPARγ KO pigs after using these cells for SCNT17. In the same year, efficient immunoglobulin gene disruption and targeted replacement in rabbits also using ZFN was reported18.
In the present study, five ApoCIII KO rabbits were generated as confirmed by PCR sequencing, using ZFN Set 1 (see Figure 1. for flow chart illustration). These animals are novel resources to study the roles of ApoCIII in lipid metabolisms.
Protocol
All animal maintenance, care and use procedures were reviewed and approved by the University Committee on the Use and Care of Animals of the University of Michigan.
1. ZFN Design and Selection
- Supply the genomic sequence information of the gene of interest (e.g. rabbit ApoCIII) to the manufacturer. Select ZFN set(s) for the experiments based on the ZFN scores.
2. Rabbit Embryo Collection
- Superovulate sexually matured (6-18 months) New Zealand White (NZW) female rabbits by subcutaneous (s.c.) injection of FSH twice/day with a dosage of 3 mg for the first two injections, 5 mg for the next two injections and 6 mg for the last two injections.
- Administer a single intravenous (i.v.) injection of 200 IU hCG at 72 hr after the first FSH injection to induce ovulation. Mate the superovulated females with a male rabbit immediately after hCG injection.
- Euthanize the superovulated donor rabbits with sodium pentobarbital (150 mg/kg, i.v.) at 16-18 hr post insemination (p.s.i.).
- Recover the oviduct ampullae by carefully collecting the entire oviducts and ovaries structure.
- Flush each oviduct with 10 ml manipulation medium of HEPES buffered TCM 199 supplemented with 10% fetal bovine serum, by injecting the medium from the uterus end of the oviduct. Collect the flushed medium at the ovary-end opening of the oviduct to a Petri dish.
- Observe and identify recovered embryos in the flushed medium, wash the embryos three times in the manipulation medium, keep them in the manipulation medium at 38.5 °C in air, and observe them under a stereomicroscope for the occurrence of fertilization. Prepare for microinjection on pronuclear stage embryos 19-21 h p.s.i.
3. Preparation of ZFN mRNA
- Purify the resulting mRNA before resuspension in RNAse-free 0.1X TE (1 mM Tris-Cl pH 8.0, 0.1 mM EDTA). Measure the concentration and store the mRNA at -80°C until use.
- Prepare the mRNAs encoding ZFN pair (4-10 ng/μl in 1 mMTris-Cl, 0.1 mM EDTA at pH 7.5), aliquot into 10 μl vials. Store them at -80 ºC until ready for use. Before microinjection, thaw the RNA vial(s), and keep it on ice.
4. Embryo Microinjection
- Prepare the microinjection platform by placing a 5 μl drop of manipulation medium on a 1-well chamber slide that has the media chamber removed. Cover the medium drop with mineral oil and place on the heated microscope stage.
- Place holding pipette (120 μm diameter) into the holder arm of the micromanipulator and position it into the drop of manipulation medium.
- Fabricate injection micropipettes by heating and pulling borosilicate glass capillary tubes in a micropipette puller device. Load the mRNA to be injected in the injection pipette.
- Turn on the compressed air supply that is connected to a microinjector.
- Place injection pipette into the injection holder, put it on the micromanipulator and position it into the drop of manipulation medium.
- Setup the microinjector, with the following suggested parameters: holdP=2 p.s.i., InjP=20 p.s.i., ClearP= 60 p.s.i., Injtime= 1 sec, which will result in an injection volume of 1-5 pl.
- Open the tip of the injection pipette by gently tapping it against the holding pipette.
- Transfer embryos (30-50) to the micromanipulation drop on the platform. Use the holding pipette to capture an embryo and align the injection needle, embryo and the holding pipette along the x-axis.
- Under 400X magnification, advance the injection pipette through the embryo. Be careful to avoid the nucleus. Once the plasma membrane is pierced, press foot pedal to inject. After injection, withdraw the needle, release the embryo. Repeat the process for all remaining embryos.
- Wash injected embryos three times in embryo culture medium, which consists of Earle's Balanced Salt Solution (EBSS) supplemented with nonessential amino acids (NEAA), essential amino acids (EAA), 1 mM L-glutamine, 0.4 mM sodium pyruvate, and 10% FBS.
- Incubate embryos at 38.5 °C in 5% CO2 in air for 1-2 hr before they are surgically transferred into the oviducts of a synchronized recipient female NZW rabbit (see step 5.1). Alternatively, culture the embryos until they reach blastocyst stage before they are used for in vitro validation or assays.
5. Embryo Transfer
- Select a female NZW rabbit 5-12 months old, in good health, weighing approx. 4.0 to 5.0 kg as recipient animal. Administer Gonadotropin Releasing Hormone (GnRH) injection intramuscularly (i.m., 50 mcg/ml, 0.3 ml/animal) to the recipient at the same time point with that of hCG injection to the donor animal(s) (see step 2.2).
- After completion of microinjection, prepare the recipient rabbit for embryo transfer (normally 16-24 hr after GnRH injection). Anesthetize the rabbit with 10-40 mg/kg ketamine (i.m.), and/or vaporized isoflurane (2-4%) through inhalation. Protect the rabbit’s eyes from excessive drying by the application of a sterile ophthalmic ointment. Observe the animal until it is fully unconscious, i.e. does not respond to pedal reflexes; squeezing the foot pad is used to check for lack of pedal reflex which is an indication of adequate anesthesia.
- Shave the rabbit's abdomen, wash with antiseptic scrub, followed by antiseptic solution, and wipe the shaved area with sterile gauze sponges.
- Perform surgery under sterile conditions, record vital signs of the recipient rabbit approximately every 15 min throughout the surgery.
- Transfer 10-25 embryos to one recipient animal, depending on the available number of embryos.
- At the end of the surgical procedures, close the muscle layer with coated absorbable suture. Suture the skin with either a subcuticular suture using absorbable suture or with simple interrupted skin sutures using nonabsorable suture (e.g. monofilament nylon or similar).
- Keep the animal warm and monitor it until its sternal recumbency is attained.
- Monitor the animal daily. Follow veterinarian’s instructions to care for the animal. Administer meloxicam (s.c. 1.0 mg/kg) to relieve pain and/or reduce fever daily post operation if determined necessary by the veterinarian.
- Remove nonabsorable skin sutures at about 10-14 days post operation.
6. Detection of ZFN Induced Gene Disruption
- For in vitro validation, perform genotyping on the in vitro cultured embryos. Lyse individual embryos that developed to morula/blastocyst stages in 5 μl NP40 solution (1% NP40 plus 1 ug/ml Proteinase K in Taq Buffer) for 0.5-1 hr at 55 °C, and 10 min at 95 °C.
- For genotyping of the offspring, collect ear skin samples from newborn kits, extract genomic DNA, and use them as templates for PCR sequencing. Apply Kwik-Stop to the sampling area after collection to stop bleeding and reduce discomfort.
- Use the lysis from step 6.2 or 6.3 as template for PCR using LA-Taq and PCR primer pair, which are located upstream (5’- TGAGGCCGGGAAGGGAGCAGTCG-3’) and downstream (5’-GCCAGGCCCACCCACGGAACAGC-3’) of the ZFN target site.
- Purify PCR products and sequence them with sequencing primer (5’-TCTGCACGCTTGGGGCTGGAG-3’).
- Identify mutant samples by looking for double curve in the sequence diagram around the targeting site. Label the ones with double curves as "positive".
- Clone the "positive" PCR products into TA cloning vector, pick up 10-20 clones, sequence the inserts, and confirm the mutations around the targeting site.
Representative Results
In vitro validation of ZFN pairs
Sixteen ZFN pairs were initially designed. The targeted disruption sequence of Set 1 is located at Exon 2 of rabbit ApoCIII (Figure 2A). Three pairs (Set 1, 2, and 3, Figure 2A) were selected and subjected to the yeast MEL-1 reporter assay to determine the ZFN activities (Figure 2B). Set 1 has a ZFN score of 224.2%, higher than those of Set 2 (196.9%) and Set 3 (177.8%) and much higher than the selection threshold (i.e. 50% of the internal positive control). Therefore Set 1 was selected for in vitro validation experiments.
A particular set needs to result in a positive rates in the embryos of 10% or higher to pass the in vitro embryo validation, based on the internal validation criteria. The mRNA (5 µg/ml) of Set 1 was microinjected to the cytoplasm of 35 pronuclear stage rabbit embryos (Figure 3A). Flushed embryos without microinjection treatment (n=31) were used as the control group. Eighteen embryos of the microinjected group developed to BL stage on D5, of which sixteen were sequenced, and eight (BL#apoc-3, 4, 5, 7, 11, 13, 15, 17, Figure 3B, red underlined) displayed mutated sequences at the targeting site (black-boxed, Figure 2B, top row, apoC3wt.seq). The BL rate of the microinjected group (51.4%) is lower than that of the control group (77.4%), indicating slightly impaired developmental competence of microinjected embryos. The positive mutation rate of the microinjected embryos is 50.0% (8/16), much higher than the selection threshold (i.e. 10%). These results revealed that Set 1 is an effective ZFN set to induce mutations at the targeting site of rabbit ApoCIII. Therefore Set 1 was chosen for in vivo production of ApoCIII KO rabbits.
Production of ApoCIII KO rabbits
The mRNA of ZFN Set 1 was microinjected to the cytoplasm of rabbit pronuclear stage embryos, and transferred 145 microinjected embryos to 7 pseudo-pregnant recipient rabbits (Figure 4A). Freshly flushed embryos (n=75) without ZFN microinjection were transferred to recipients (n=6) as the control group. After 30 days of gestation, 21 kits were born in the microinjected group. PCR sequencing identified five (apoC3-R1, R9, R11, R12 and R16) as positive KO kits (Figure 4B). The term rate calculated as total term kits/total embryos is 14.5% (21/145), whereas the rate is 29.3% (22/75) in the control group. The KO rate calculated as total KO kits/total term is 23.8% (5/21). One KO kit (apoC-R16) died five days postpartum (20.0%, 1/5). The indelsat the targeting site include two insertions and three deletions; they areΔ1, +1, +1, Δ20 and Δ21, for Rabbits # R1, R9, R11, R12, and R16, respectively (Figure 4B).
Bioinformatics analysis was used to identify potential ZFN off-targets with 7 or fewer mismatches to Set 1 sequence, as described previously17. Only one predicted possible off-target site, located on Chromosome 14, was identified with 7 mismatched bps. PCR assay detected no off-target events in any of the founder animals.
Discussion
In the present work, five ApoCIII KO rabbits were generated using the ZFN technology. Previously the only report of the production of GTT rabbits also used the ZFN technology18. The present work confirmed that ZFN is useful to effectively target genes in rabbits.
In the present study, the transgenic rate (positive kits/total born) is23.8% (5/21), which is comparable to that of the previous ZFN report in rabbits (30.8%, 16/52)18, and those reported of using ZFN in other animal species, including zebra fish19, mice20, rats21 and pigs17. This rate is in fact higher than the rates of many conventional transgenic rabbit production studies, which normally fall in the range of 5-20%. For example, in an effort to product ApoCIII transgenic rabbits via conventional DNA microinjection, Ding et al. obtained 3 positive founders out of 54 pups born (5.6%)22.
Consistent with previous findings, the mutations at the targeting sites are variable, including deletions or insertions consisting different number of bases (ranging from 1-21 in the five KO rabbits). Based on the specific sequence information of the founder animals, it is predicted that at least four (i.e. those containing Δ1, +1, +1, or Δ20 mutations) would have function loss of ApoCIII. Animal R-16 with Δ21 may not display functional loss, as this mutation is predicted to only cause loss of seven amino acids, but not a reading frame shift. Ultimately, phenotype assays are necessary to determine if these founder animals truly display the loss of ApoCIII functions.
None of the five KO rabbits generated in the present study contain biallelic modifications. Interestingly, this is also the case in the previous ZFN rabbit report. In contrast, when ZFNs targeting α1,3-galactosyltransferase were applied to pig cells, the frequency of targeting a single allele ranged from 7-46%, with approximately one-third of mutations creating single-step biallelic knockouts 23. Consistent with this, when TALEN was applied to pig fibroblast cells, the biallelic modifications were found in approximately 15-40% of the total positive clones14. It is possible that the failure to generate biallelic KO rabbits in the present study may indicate a species difference (i.e. rabbits vs. pigs) for such capacity of nuclease based gene targeting. It is, however, more likely that this is simply because the number of KO rabbits generated in this project is relatively small. We believe that ZFN is capable of generating bialleic mutations in rabbits, which should be considered as another potential advantage of ZFN based GTT rabbit production. Further experiments are needed to confirm such capacity of ZFN in rabbits.
In conclusion, the present work demonstrates that ZFN based gene targeting approach is effective in producing KO rabbits. In particular, we generated five ApoCIII KO rabbits with a satisfactory transgenic rate of 23.8%. These animals are believed to provide more meaningful information about this protein’s role on lipid metabolisms in humans than the corresponding mouse models. We predict ZFN based gene targeting, as well as other nuclease based technologies such as TALEN and RGEN, in nonmurine animals will significantly facilitate the development of novel animal models to study various human diseases.
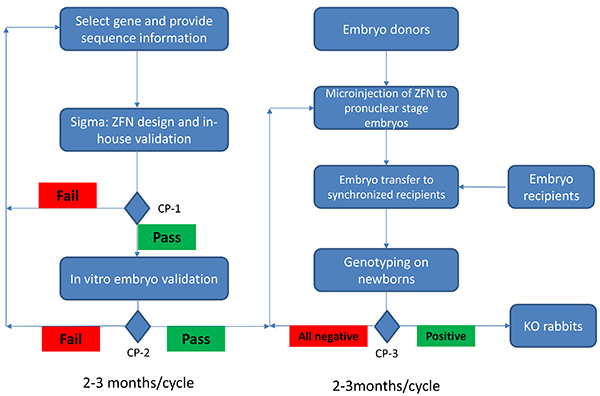
Figure 1. Flow chart of production of knockout rabbits using ZFN technology. Production of KO rabbits using ZFN technology starts with the selection of the gene of interest. The sequence information is provided, ZFN sets are designed, and subjected to yeast based in-house validation. At check point (CP) 1 (CP-1), only those sets passed in-house validation (50% or higher of the internal positive control) will be provided to the users for selection. If no sets pass CP-1, additional sequence may be needed for designing effective ZFN sets. An in vitro embryo validation is followed to ensure the selected ZFN set can induce mutations at selected sites (CP-2). ZFN set(s) will only be used if it passes CP-2 (>=10% mutation rates in in vitro embryos). Failure at CP-2 will require a redesign of the ZFN sets. Embryo donors will be prepared and pronuclear stage embryos will be microinjected with the validated ZFN sets. These microinjected embryos will be transferred to synchronized embryo recipients. After one month gestation period, newborns will be genotyped (CP-3). If none of the newborns are positive, additional microinjection will be performed. It takes 2-3 months from start of the project to CP-2, assuming no failure during the process. It takes additional 2-3 months from CP-2 to CP-3. Therefore it is possible to generate a knockout rabbit in a 4-6 month time frame using the ZFN technology. Click here to view larger image.
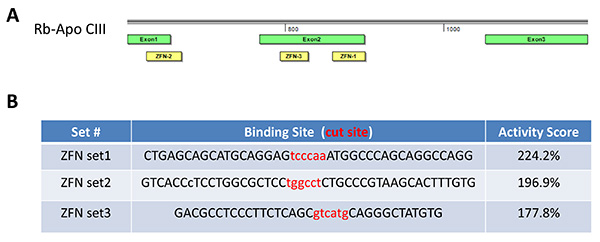
Figure 2. ZFN design. Three ZFN sets (Set 1, 2, and 3) were designed targeting different sequences on Exon 1 or Exon 2 of rabbit ApoCIII (A). All three sets were subjected to the yeast MEL-1 reporter assay to determine the ZFN activities. According to the manufacture’s protocols, ZFNs that show >50% activities of that of the manufacture’s internal positive control are regarded as useful for in vitro and in vivo genome editing experiments. The ZFN activities were 224.2% for Set 1, 196.9% for Set 2 and 177.8% for Set 3 (B), therefore Set 1 was selected in the present study. Click here to view larger image.
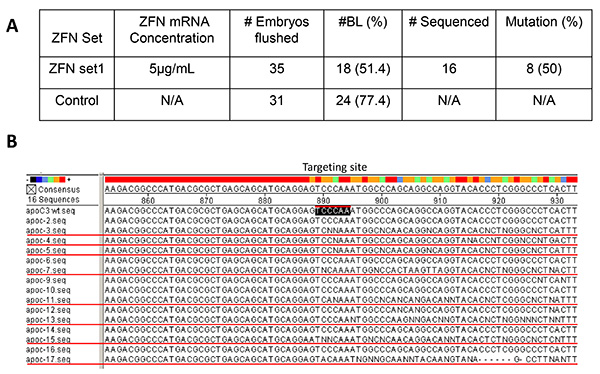
Figure 3. In vitro embryo validation of ZFN. The mRNA (5 μg/ml) of Set 1 was microinjected to the cytoplasm of 35 pronuclear stage rabbit embryos (A). Flushed embryos without microinjection treatment (n=31) were used as the control group. The BL development rate was 77.4% in the control group. In the microinjected group, the BL rate was 51.4%. Of 16 BLs subjected to PCR sequencing, 8 (50%) were identified as positive, indicating Set 1 is an effective ZFN set to induce mutations at the targeting site (B, apoc3wt.seq, black-boxed) of ApoCIII in rabbits. The BLs containing mutations are BL#apoc-3, 4, 5, 7, 11, 13, 15, and 17 (B, red underlined). The remaining BLs (#apoc-2, 6, 9, 10, 12, 14 and 16) do not have mutations at the targeting site. Click here to view larger image.
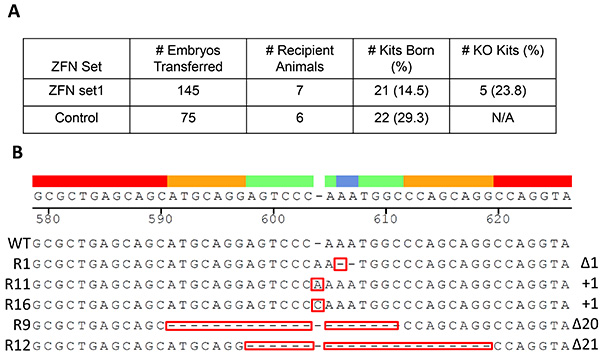
Figure 4. Production of ApoCIII KO rabbits. One hundred and forty-five embryos microinjected with ZFN Set 1 mRNAs were transferred to 7 pseudo-pregnant recipient rabbits (A). Freshly flushed embryos (n=75) without ZFN microinjection were transferred to recipients (n=6) as the control group. The term rate calculated as total term kits/total embryos in experiment group is 14.5% (21/145), whereas the rate is 29.3% (22/75) in the control group (A). Out of 21 kits born in the microinjected group, five (R1, R9, R11, R12 and R16) were identified as positive KO kits after PCR sequencing (B). The indels at the targeting site include two insertions (+1 for both R9 and R11) and three deletions (Δ1, Δ 20, Δ 21 for R1, R12 and R16, respectively). Click here to view larger image.
開示
The authors have nothing to disclose.
Acknowledgements
This work was partially funded by grants from the National Institutes of Health (HL105114, HL088391, NS066652, and HL068878 to Y.E.C), the American Heart Association (National Scientist Development 0835237N to J.Z.). Y.E.C. is financially supported as endowed Frederick Huetwell Professor of Cardiovascular Medicine at the University of Michigan Medical Center (UMMC). This work utilized Core Services supported by Center for Advanced Models for Translational Sciences and Therapeutics (CAMTraST) at UMMC.
Materials
| APOC3-ZFN | Sigma Aldrich | CSTZFNY-1KT | Target Gene: APOC3, Lot Number: 12201118MN |
| mMESSAGE kit | Invitrogen | AM1344M | mRNA synthesis |
| MEGAclear Kit | Invitrogen | AM1908M | mRNA purification |
| Follicle-stimulating hormone | Bioniche Life Sciences | Folltropin-V | Treating embryo donor rabbits |
| Human chorionic gonadotropin | Intervet | Chorulon | Treating embryo donor rabbits |
| Gonadotropin-releasing hormone | Prospecbio | HOR-255 | Treating embryo recipient rabbits |
| Earle's Balanced Salt Solution (EBSS) | Thermo Fisher Scientific | SH30029.02 | Embryo culture, base medium |
| MEM (nonessential amino acid) | Sigma Aldrich | M7145 | Embryo culture, supplements |
| BME AMINO ACIDS solution | Sigma Aldrich | B6766 | Embryo culture, supplements |
| Glutamine | Gibco | 25030-149 | Embryo culture, supplements |
| Sodium pyruvate | Gibco | 11360-070 | Embryo culture, supplements |
| Fetal bovine serum | Sigma Aldrich | 12003C | Embryo culture, supplements |
| HEPES buffered TCM 199 | Gibco | 12350039 | Embryo manipulation medium |
| Incubator | Eppendorf | Galaxy 170 | Embryo culture equipment |
| Micromanipulator | Eppendorf | TransferMan NK 2 | Embryo manipulation equipment |
| Micropipette puller | Sutter Instruments Inc. | P-1000 | Embryo manipulation equipment |
| Microinjector | Tritech Research | MINJ-D | Embryo manipulation equipment |
| Borosilicate glass capillary tubes | World Precision Instruments, Inc. | TW100F-6 | Embryo manipulation supply |
| Euthasol (pentobarbitol sodium) | Virbac AH, Inc. | ANADA#200-071 | Euthanization of embryo donor rabbits |
参考文献
- Ooi, E. M., Barrett, P. H., Chan, D. C., Watts, G. F. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clinical Science. 114, 611-624 (2008).
- Pollin, T. I., et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322, 1702-1705 (2008).
- Ginsberg, H. N., et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. The Journal of Clinical Investigation. 78, 1287-1295 (1986).
- Cohn, J. S., et al. Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis. 177, 137-145 (2004).
- Cohn, J. S., Patterson, B. W., Uffelman, K. D., Davignon, J., Steiner, G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. The Journal of Clinical Endocrinology and Metabolism. 89, 3949-3955 (2004).
- Maeda, N., et al. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. The Journal of Biological Chemistry. 269, 23610-23616 (1994).
- Jong, M. C., et al. Apolipoprotein C-III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. Journal of Lipid Research. 42, 1578-1585 (2001).
- James, J. F., Hewett, T. E., Robbins, J. Cardiac physiology in transgenic mice. Circ. Res. 82, 407-415 (1998).
- Duranthon, V., et al. On the emerging role of rabbit as human disease model and the instrumental role of novel transgenic tools. Transgenic Research. 21, 699-713 (2012).
- Fan, J., Watanabe, T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 99, 261-282 (2003).
- Shiomi, M., Ito, T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis. 207, 1-7 (2009).
- Morehouse, L. A., et al. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. Journal of Lipid Research. 48, 1263-1272 (2007).
- Getz, G. S., Reardon, C. A. Animal models of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 32, 1104-1115 (2012).
- Carlson, D. F., et al. Efficient TALEN-mediated gene knockout in livestock. Proceedings of the National Academy of Sciences of the United States of America. , (2012).
- Jiang, W., Bikard, D., Cox, D., Zhang, F., Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnology. 31, 233-239 (2013).
- Petersen, B. Update on ‘molecular scissors’ for transgenic farm animal production. Reproduction, Fertility, and Development. 25, 317-318 (2012).
- Yang, D., et al. Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Research. 21, 979-982 (1038).
- Flisikowska, T., et al. Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS ONE. 6, e21045 (2011).
- Foley, J. E., et al. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nature Protocols. 4, 1855-1867 (2009).
- Carbery, I. D., et al. Targeted genome modification in mice using zinc-finger nucleases. 遺伝学. 186, 451-459 (2010).
- Mashimo, T., et al. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS ONE. 5, e8870 (2010).
- Ding, Y., et al. Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Research. 20, 867-875 (2011).
- Hauschild, J., et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 108, 12013-12017 (2011).

