Microinjection Wound Assay and In vivo Localization of Epidermal Wound Response Reporters in Drosophila Embryos.
概要
The embryonic epidermis of very late stage Drosophila embryos provides an in vivo system for rapid puncture wound response analysis and can be combined with genetic manipulations or chemical microinjection treatments to advance studies in wound healing for translation into mammalian models.
Abstract
The Drosophila embryo develops a robust epidermal layer that serves both to protect the internal cells from a harsh external environment as well as to maintain cellular homeostasis. Puncture injury with glass needles provides a direct method to trigger a rapid epidermal wound response that activates wound transcriptional reporters, which can be visualized by a localized reporter signal in living embryos or larvae. Puncture or laser injury also provides signals that promote the recruitment of hemocytes to the wound site. Surprisingly, severe (through and through) puncture injury in late stage embryos only rarely disrupts normal embryonic development, as greater than 90% of such wounded embryos survive to adulthood when embryos are injected in an oil medium that minimizes immediate leakage of hemolymph from puncture sites. The wound procedure does require micromanipulation of the Drosophila embryos, including manual alignment of the embryos on agar plates and transfer of the aligned embryos to microscope slides. The Drosophila epidermal wound response assay provides a quick system to test the genetic requirements of a variety of biological functions that promote wound healing, as well as a way to screen for potential chemical compounds that promote wound healing. The short life cycle and easy culturing routine make Drosophila a powerful model organism. Drosophila clean wound healing appears to coordinate the epidermal regenerative response, with the innate immune response, in ways that are still under investigation, which provides an excellent system to find conserved regulatory mechanisms common to Drosophila and mammalian epidermal wounding.
Introduction
Manipulation of Drosophila embryos using a microinjection technique is a well-established assay1. The significance of the epidermal wound response reporter method is to combine the protocol for puncture/microinjection with fluorescent reporters and visualize an in vivo response to epidermal injury in Drosophila embryos. The goal of this method is to enable a broader set of scientists to use Drosophila as a tool to investigate the processes that regulate the transcriptional response to epidermal puncture injury. The single-layered epidermis in Drosophila provides a simple system to study an epidermal wound response after a puncture injury2. The Drosophila embryo is a robust model system to genetically dissect the stages of wound repair, including wound response, inflammation, and reepithelialization3. This is in part because many or most zygotic mutants survive to the end of embryogenesis and develop epidermal barriers even when developmental patterning goes profoundly awry. Previous characterization of a well-conserved transcription factor Grainy head (Grh), identified that Grh-target genes Dopa decarboxylase (Ddc) and tyrosine hydroxylase (ple) are transcriptionally activated around the sites of injury4. Drosophila as a model organism for wound response provides a complementary line of investigation to advance studies in mammalian wound healing5. Subsequent studies have identified additional Drosophila wound-induced genes and developed a "toolkit" of many fluorescent wound reporters to monitor the in vivo epidermal wound response to clean wounding6. Recent reports on the regulation of Drosophila wound healing have focused on the wound closure phenotype, allowing discovery of many pathways regulating cell migration7,8. With the analysis of the fluorescent wound reporters, our studies have identified a novel set of genes required for the localized expression of epidermal wound-inducible genes9. One of the merits of the wound response reporter method is that the results provide more insight into a mechanism of how signals are transduced from the site of injury to the neighboring cells. However, one of the demerits of the wound response reporter method is that the results do not directly link to phenotypes in wound healing or repair. Broader use of the clean wound puncture protocol in genetic and chemical screens will allow new regulators of the transcriptional response to epidermal wounding to be identified and further the translation of wound healing discoveries into mammalian models of injury treatments.
Protocol
The Drosophila embryonic wound protocol can be summarized in five steps: (1) Embryo Collection, (2) Embryo Preparation (3) Embryo Wounding, (4) Embryo Microinjection, and (5) Embryo Fixation.
1. Embryo Collection
- Collect adult flies, 2-3 days after eclosing, add 50 females and 25 males to an embryo collection cage.
- Place a fresh apple juice agar plus yeast plate each morning for 3 days.
Notes: For optimal embryo collection, cycle the flies on a 12 hr diurnal schedule in a 25 °C incubator (05:00 lights "ON" and 17:00 lights "OFF"); female flies respond to changes in the light cycle and will deposit larger quantities of eggs during a switch from lights "ON" to lights "OFF"10,11. - In the afternoon of Day-3, place a fresh apple juice plus yeast agar plate on the cage and collect embryos for 2 hr. Place a new yeast plate on the cage and save the 2 hr collection plate.
- Store the collection plate for an additional 14 hr at 25 °C to age the embryos.
Notes: Wound healing can be assayed at any stage during Drosophila development; the wound-induced transcriptional reporters described in this paper have an optimal activity between stages 15-16 12. By late stage 17, the embryo is too old to easily pierce the maturing cuticle and efficiently activate the wound reporters or detect wound-induced transcription. To allow the collection time and the wounding time to occur during peak lab hours, we follow a schedule of embryo collection for 2 hr (16:00-18:00), exchange the collection plate with a new plate at 18:00, age the collection plate at 25 °C for 14 hr (overnight), and wound the embryos at 08:00 (next day).
2. Embryo Preparation
Notes: This protocol includes the use of many tools and objects that are sharp or made of glass. Handle all sharps and glass objects with care to avoid personal injury.
- Gently remove embryos from collection plates with a paintbrush by adding 2-3 ml water to plate and swirling. Transfer water and embryos from plate to nylon mesh and collection tube.
- Add 4-5 ml of bleach to the cap of a plastic Petri dish plate and soak the mesh covered end of the collection tube containing the embryos in bleach for 2 min; which removes the outer eggshell of the embryo. Manually rotate the collection tube a couple of times to prevent embryos from clumping in the bleach. Rinse embryos with water 10x and dry the mesh covered collection tube containing the dechorionated embryos on a paper towel.
- Use a dissecting needle to transfer ∼50 embryos from nylon mesh to an agar plate. We add green food coloring to the agar plate to add contrast and aid in embryo alignment. Under a dissecting microscope, make two parallel rows of ∼25 embryos, aligning the embryos on the slide so that they will be at right angles to the puncture needle on the microinjection apparatus. Leave a little space (about 1 embryo length) between the embryos for gas exchange purposes. The two parallel rows should be approximately 5 mm apart.
- Place a slide with double stick tape on top of the aligned embryos and carefully press the slide to transfer embryos from the green plate to the slide. Then air dry the embryos ∼25 min to reduce internal pressure on the embryo and prevent a burst when wounding.
- Place 2-3 drops of halocarbon oil mix over the embryos to prevent further dehydration.
3. Embryo Wounding
- Place the embryo slide in the injection microscope stage. Find the embryos in the field of view and practice moving the stage of the inverted microscope.
Notes: Before working with the needle, focus on middle plane of embryo along the dorsoventral axis. To allow for efficient wounding of the aligned embryos, begin wounding the row closer to the needle apparatus. Move the stage to bring the top of the row of aligned embryos into the field of view. - Carefully place and secure a pulled-needle in the needle holder. Use the micromanipulator to move the needle down toward slide. Align the needle with the embryo, in same focal plane. Once the needle is in focus with the embryo, do not move the needle with the micromanipulator.
- Move the stage to puncture the embryo through and through, then move to the next embryo in the row. When done wounding the first row, use the micromanipulator to move the needle completely up and away from the stage before moving the slide; manually rotate the slide 180° and wound the second row.
- Store the embryos under halocarbon oil at room temperature and proceed with embryo fixation if doing in situ hybridization or antibody staining (see Procedure step 5). Alternatively, store the embryos for 4-6 hr under halocarbon oil and proceed with wound transcriptional fluorescent reporter visualization (Representative Results).
Notes: For the visualization of the fluorescent reporter, we anesthetize the embryos using 50% 1-phenoxy-2-propanol13. The embryos are removed from the halocarbon oil and placed on a new slide. We place glass beads around the embryos, add a few drops of the anesthetic, and place a cover slip on the embryos.
4. Embryo Microinjection
Notes: The microinjection apparatus that we use in the protocol is not automated to deliver specific volume during the assay. We add a dye to the solution to visualize the microinjection and try to normalize the volume delivered. If too much solution is injected into the embryo, the vitelline membrane will burst.
- Load needle from the rear with 1 µl of chemical solution plus dye (e.g. 0.6 M H2O2). Allow capillary action to draw the chemical solution to the tip of the needle.
- To break a needle, place a cover slip at an angle on a slide and coat the edge with halocarbon oil mix. Bring the mounted needle into focus with the edge of the angled cover slip and gently move cover slip edge across the tip of the needle until broken. Apply pressure on microinjection apparatus and check that the chemical solution plus dye is flowing out the needle.
- Place the slide containing aligned embryos on the microscope stage. Proceed to focus the embryo and the needle in the same plane. Simultaneously puncture and microinject chemical solution plus dye into each embryo.
Notes: Clogged needles occur frequently; break a new needle to avoid incomplete microinjection. A blunt needle tip does not wound as cleanly as an angled needle tip. Adjusting the coverslip to a non-90° angle during breakage is optimal to produce an angled needle tip.
5. Formaldehyde Fixation
Notes: The chemicals used in this Drosophila fixation are harmful. Use personal protective equipment (e.g. gloves, laboratory coat, and safety goggles) when handling the chemicals. Follow individual institutional guidelines for disposal of regulated chemicals.
- After wounding, wait 15, 30, 60, or more minutes for the transcriptional activation of wound-induced genes to occur. To remove embryos from injection slides, carefully drain the halocarbon oil mix from the slides, lean the slide against a rack and blot the end with a Kimwipe.
- Use a glass pipette to rinse heptane over the slide and wash the rinse liquid into a glass Petri dish. Heptane will dissolve the tape and release the embryos. Continue to rinse until all the embryos are removed from the slide. Proceed to the next slide, until all embryos are removed from slides.
- Transfer the heptane plus embryo solution from the glass dish into a 20 ml scintillation vial. Shake the vial for 2-3 min, remove the heptane and add 5 ml of fresh heptane.
- Add 5 ml of fresh, fixation solution (see reagents list for recipe). Cap the vial and attach to an orbital platform shaker. Shake the vial containing the embryos for 25 min at 220-230 rpm.
- After shaking, let the bubbles at the interface pop. Completely remove the bottom, aqueous phase with a pipette, avoiding pulling up the embryos. The bottom layer consists of the fixation solution and should be disposed properly.
- Add 5 ml of methanol, cap the vial and shake vigorously by hand for 20-30 sec, swirl and place it on the bench. Wounded embryos will stay at the interphase and will not sink to the bottom.
Notes: After fixation the vitelline membrane of an unwounded embryo will normally burst during the dehydration step with methanol. However, the vitelline membrane in a wounded embryo is already broken and the dehydration step will not remove the vitelline membrane. Hand-devitellinzation is required to complete the fixation protocol. - Carefully remove the top heptane layer and then add 5 ml of fresh methanol. Shake the vial and the embryos should sink.
- Remove the methanol plus embryo solution with a glass transfer pipette and collect in a nylon mesh collection tube in a glass dish.
- Remove the methanol and rinse the embryos with 1x PBS solution.
- Transfer the embryos from the nylon mesh to an apple juice plate. Spread the embryos out along the surface of the plate.
- Transfer the embryos to a double stick tape glass slide. Cover the embryos with 1x PBS.
Notes: The embryos tend to clump because of the vitelline membrane. Using an apple juice place allows for quick separation of the clumped embryos. The embryos must be removed from the plate and transferred to a double stick tape glass slide because embryos sink into the agar plate when force is applied to remove the embryo from the vitelline membrane. - Carefully push the embryos with a dissecting needle to "pop" the vitelline membrane. The embryo should sink in the PBS.
- Use a glass pipette to transfer the 1x PBS plus embryos to a 1.5 ml tube. Rinse the embryos with 1x PBS and store at 4 °C. Proceed to dehydrate the embryos using an Ethanol:PBS series and continue with in situ hybridization or immunolocalization protocol14.
Representative Results
To obtain the results shown in this method, the open reading frame of green fluorescent protein (GFP) is fused to a promoter/wound-induced enhancer sequence from Dopa Decarboxylase (Ddc) and DsRed is fused to a wound enhancer sequence from tyrosine hydroxylase (ple)4,6. In a live Drosophila embryo, maturation of the fluorescent wound reporter protein is optimal 4-6 hr after wounding, which allows time for the accumulation of sufficient protein to visualize, as well as time for the fluorescent protein to oxidize to the fluorescent state15. To observe the reporter localization, a compound fluorescent microscope is sufficient. To observe a higher magnification of the reporter localization, a confocal microscope is optimal to generate a maximum projection of multiple optical sections (Figure 1). Confocal in vivo imaging of Drosophila embryos is complicated by the rapid undulations of the embryo. . A full-view of embryo can be obtained with 20X objective and a close-up view of wound site can be obtained with 40X objective. A top-view of epidermal wound reporter localization can be imaged with 10 successive 1µm optical sections. A lateral-view of epidermal wound reporter localization can be imaged with 40 successive 1 µm optical sections.
Using standard genetic crossing methods, it is possible to combine wound reporters with genetic mutations. One example presented in this paper is Flotillin-2 (Flo-2), because loss-of-function (null allele generated with P-element insertion) and gain-of-function (overexpression generated with ubiquitous expression in all cells) mutants of Flo-2 demonstrate the Flo-2 gene product is necessary and sufficient to inhibit the localization of the Ddc-GFP wound reporters9 (Figures 2A and 2B). Using the microinjection protocol, it is possible to test whether introduction of chemical solutions superactivates or inhibits the localization of wound reporters. Chemically-wounded embryos were simultaneously wounded and injected with a 1:4 ratio of 1% toluidine blue dye and solubilized compounds. Toluidine blue dye allowed for visual confirmation of solubilized compounds being injected into the body cavity. Control embryos were wounded with a broken needle containing 1:4 ratio of 1% toluidine blue dye and solute without chemical. Two examples presented in this paper are hydrogen peroxide (H2O2) and methyl-β-cyclodextrin (MβCD), because both chemical solutions are sufficient to globally activate the localization of the Ddc-GFP wound reporters9 (Figures 2C and 2D). Hydrogen peroxide (H2O2) was diluted in H2O to 0.6 M. Methyl-β-cyclodextrin (MβCD) was solubilized in 1 mM NaOH to 3 mM. Using the embryo fixation protocol, it is possible to detect the transcription activation of wound response genes in a localized domain, surrounding a wound site. One example presented in this paper is the in situ hybridization of RNA probes to detect Ddc and ple transcripts4,6,9 (Figures 3A and 3B).
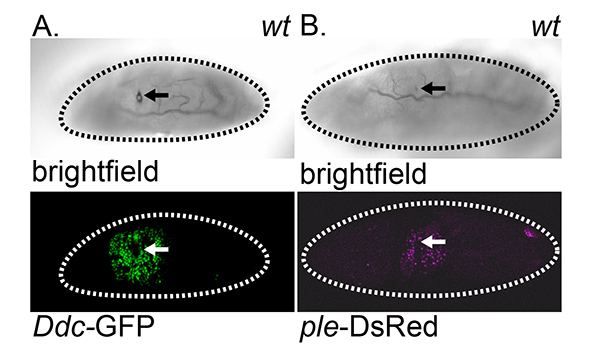
Figure 1. Ddc-GFP and ple-DsRed wound reporter localization. Brightfield and fluorescent images in wounded wild type embryos. (A) Top-view of Ddc-GFP wound reporter. (B) Top-view of ple-DsRed wound reporter. All images were collected on a Leica SP2 confocal microscope, with 20X objective. Arrows mark site of wound. Dashed lines in the data panels mark the outlines of embryos. Click here to view larger image.

Figure 2. Localization of the Ddc-GFP wound reporter in genetic mutant backgrounds and chemical microinjected embryos. Brightfield and fluorescent images of wounded Flotillin-2 (Flo-2) mutant embryos. (A) Loss-of-function Flo-2 {KG00210} mutants, expansion of reporter activity throughout all epidermal cells. (B) Gain-of-function Flo-2 (armadillo-GAL4, UAS-Flo2) mutants, inhibition of reporter activity throughout all epidermal cells. (C) Hydrogen peroxide (H2O2) solution microinjection, expansion of reporter activity throughout all epidermal cells. (D) Methyl-β-cyclodextrin (MβCD) solution microinjection, expansion of reporter activity throughout all epidermal cells. All images were collected on a Leica SP2 confocal microscope, with 20X objective. Arrows mark site of wound. Dashed lines in the data panels mark the outlines of embryos. Click here to view larger image.
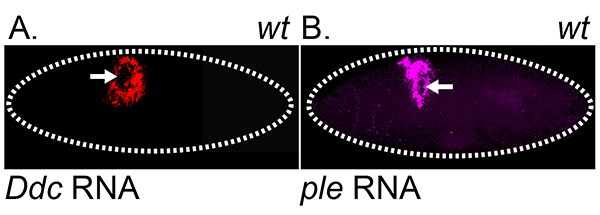
Figure 3. Detection of wound response gene RNA transcripts. Fluorescent images of in situ hybridization and RNA detection in wounded wild type embryos. Ddc (A) and ple (B) RNA transcripts accumulate around the wound site. All images were collected on a Leica SP2 confocal microscope, with 20X objective. Arrows mark site of wound. Dashed lines in the data panels mark the outlines of embryos. Click here to view larger image.
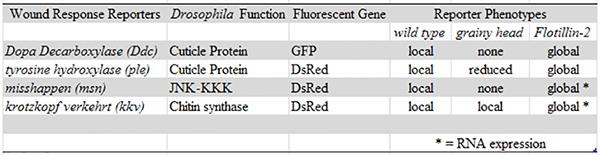
Table 1. Summary of epidermal wound response reporters. Insertions of the four wound response reporters (Ddc, ple, msn, kkv) are available on both the second and third chromosomes. All of the wound response reporters activate the fluorescence gene (GFP or DsRed) in a limited number of epidermal cells surrounding the site of puncture injury in the wild type background (e.g. "local" phenotype). Ddc and msn wound response reporters require Grh for activation of the fluorescence gene after puncture injury (e.g. "none" phenotype). All of the wound reporters require Flo-2 for localization of the fluorescence gene after puncture injury (e.g. "global" phenotype). Click here to view larger image.
Discussion
An immediate transcriptional regulatory response to external cues allows cells to coordinate a localized response to extracellular stimuli, which can include stress, damage, or infection. The improper control of a localized response can have damaging effects on neighboring cells, as well as a waste of resources to repair the injury. The Drosophila embryo provides an excellent system to conduct basic wound healing experiments in an inexpensive and easy-to-maintain model organism. The potential for collaborations between research in other model organisms and Drosophila provides a unique opportunity to explore many biological questions.
An additional technique of the wound reporters is the genetic combination of mutant backgrounds, providing an efficient method for testing the contribution of new genes to the activation of the epidermal wound response pathway. We have epidermal wound-induced transcriptional reporters available on both the 2nd and 3rd chromosomes5. In this study we use UAS and GAL4 systems of overexpression16. For studies with lethal mutant alleles we determine the genetic background using a fluorescent balancer chromosome, e.g. Kruppel-GFP17. We have analyzed the wound reporter localization in several genetic backgrounds (Table1).
A possible modification of the technique is to combine microinjection and wounding. Microinjection can be used to introduce a chemical solution into the embryo. Several chemical compounds have been tested and were found to regulate the activity of the epidermal wound reporters9. This method of simultaneous wounding and microinjection can be useful to increase the number of cells in the Drosophila embryo that respond a wound signal and can be directly used to monitor gene expression changes after wounding18. A future application of the microinjection technique will be to test novel chemicals for regulation of the epidermal wound response.
Drosophila as a model system for genetic studies offers a wide range of simple protocols to efficiently address complex questions. Recent reports on the feasibility of Drosophila techniques highlights the use of hemocyte migration19 and parasitic wasp infection20 as a means to further broaden the impact of the Drosophila research community. In addition to wound repair studies, Drosophila provides a classical model for regeneration with the imaginal disc system21,22. The combined advances in imaginal disc regeneration studies and puncture/microinjection wounding provides an excellent system to discover well-conserved components of tissue repair.
開示
The authors have nothing to disclose.
Acknowledgements
This protocol has been developed in collaboration with previous members of the McGinnis Lab, Kim A. Mace and Joseph C. Pearson. We thank the continued efforts by the Bloomington Drosophila Stock Center for their work to organize and distribute valuable stocks. This work was supported by funding from the National Institutes of Health (R01 GM077197 and K12 GM68524) and the family of Herbert Stern. MTJ is currently supported by Grant Number 5G12RR003060-26 from the National Center for Research Resources and Grant Number 8G12MD7603-27 from the National Institute On Minority Health and Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Minority Health And Health Disparities or the National Institutes of Health.
Materials
| yeast | Sigma Aldrich | 51475 | |
| apple juice | Generic | ||
| agar | Fisher Scientific | 50-824-297 | |
| bleach | Generic | ||
| halocarbon oil, 700 W | Sigma Aldrich | H8898 | |
| halocarbon oil, 27 W | Sigma Aldrich | H8773 | |
| 1-phenoxy-2-propanol | Sigma Aldrich | 484423 | |
| hydrogen peroxide | Fisher Scientific | H324 | |
| methyl-β-cyclodextrin | Sigma Aldrich | C4555 | |
| toluidine blue | Sigma Aldrich | 89640 | |
| formaldehyde, 16% | Polysciences, Inc | 18814-20 | toxic chemical |
| heptane | Fisher Scientific | H360-1 | toxic chemical |
| methanol | Fisher Scientific | A412-1 | toxic chemical |
| 10x PBS | Fisher Scientific | 50-899-90013 | |
| 0.5 M EGTA | Fisher Scientific | 50-255-956 | |
| RNase free H2O | Fisher Scientific | BP2819-100 | |
| Equipment | Company | Catalog Number | コメント |
| Drosophila incubator | Genessee Scientific | 59-197 | |
| fly cage | Genessee Scientific | 59-100 | |
| embryo collection tube | Genessee Scientific | 46-101 | |
| plastic Petri dish | Fisher Scientific | 50-202-037 | |
| double-stick tape | Generic | ||
| paintbrush | Generic | ||
| dissection needle | Fisher Scientific | 08-965A | sharp object |
| glass cover slip | Thermo Scientific | 3306 | sharp object |
| microscope slide | Thermo Scientific | 4445 | sharp object |
| dissecting microscope | Carl Zeiss | Stemi-2000 | |
| capillary needle | FHC | 30-30-1 | sharp object |
| needle puller | Narishige | PC10 | |
| microinjection needle holder | Narishige | MINJ4 | |
| micromanipulator | Narishige | MN151 | |
| inverted microscope | Carl Zeiss | Primo-Vert | |
| glass Petri dish | Fisher Scientific | 08-747A | sharp object |
| glass Pasteur pipette | Fisher Scientific | 22-063-172 | sharp object |
| transfer bulb | Fisher Scientific | 03-448-25 | |
| Kimwipe | Fisher Scientific | 06-666A | |
| scintillation vial | Fisher Scientific | 03-337-7 | sharp object |
| orbital shaker | Fisher Scientific | 14-259-260 | |
| 1.5 ml tube | Fisher Scientific | 05-408-129 | |
| laser scanning confocal | Leica | SP2 | |
| fixation solution | (5 ml) | ||
| 16% formaldehyde | 2.5 ml | toxic chemical | |
| 10x PBS | 0.5 ml | ||
| 0.5 M EGTA | 0.5 ml | ||
| RNase free H2O | 1.5 ml | ||
| Halocarbon oil mix | (10 ml) | ||
| halocarbon oil, 700 W | 5 ml | ||
| halocarbon oil, 27 W | 5 ml |
参考文献
- Germeraad, S. Genetic transformation in Drosophila by microinjection of DNA. Nature. 262 (5565), 229-231 (1976).
- Payre, F. Genetic control of epidermis differentiation in Drosophila. Int. J. Dev. Biol. 48 (2-3), 207-215 (2004).
- Gurtner, G. C., Werner, S., Barrandon, Y., Longaker, M. T. Wound repair and regeneration. Nature. 453 (7193), 314-321 (2008).
- Mace, K. A., Pearson, J. C., McGinnis, W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 308 (5720), 381-385 (2005).
- Ting, S. B., Caddy, J., et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 308 (5720), 411-413 (2005).
- Pearson, J. C., Juarez, M. T., Kim, M., Drivenes, &. #. 2. 1. 6. ;., McGinnis, W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106 (7), 2224-2229 (2009).
- Campos, I., Geiger, J. A., Santos, A. C., Carlos, V., Jacinto, A. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. 遺伝学. 184 (1), 129-140 (2010).
- Lesch, C., Jo, J., Wu, Y., Fish, G. S., Galko, M. J. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. 遺伝学. 186 (3), 943-957 (2010).
- Juarez, M. T., Patterson, R. A., Sandoval-Guillen, E., McGinnis, W. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet. 7 (12), e1002424 (2011).
- Allemand, R. Influence of light condition modification on the circadian rhythm of vitellogenesis and ovulation in Drosophila melanogaster. J. Insect Physiol. 22 (8), 1075-1080 (1976).
- Allemand, R. Rhythm of vitellogenesis and ovulation in photoperiod ld 12:12 of Drosophila melanogaster. Insect Physiol. 22 (7), 1031-1035 (1976).
- Pierre, S. E., Thurmond, J. FlyBase 101 – the basics of navigating FlyBase. Nucleic Acids Res. 40 (D1), D706-D714 (2012).
- Wyeth, R. C., Croll, R. P., Willows, A. O. D., Spencer, A. N. 1-Phenoxy-2-propanol is a useful anaesthetic for gastropods used in neurophysiology. J. Neurosci. Methods. 176 (2), 121-128 (2009).
- Kosman, D., Mizutani, C. M., Lemons, D., Cox, W. G., McGinnis, W., Bier, E. Multiplex detection of RNA expression in Drosophila embryos. Science. 305 (5685), 846 (2004).
- Barolo, S., Castro, B., Posakony, J. W. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. BioTechniques. 36 (3), 436-440 (2004).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Casso, D., Ramírez-Weber, F., Kornberg, T. B. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech. Dev. 91 (1-2), 451-454 (2000).
- Patterson, R. A., Juarez, M. T., Hermann, A., Sasik, R., Hardiman, G., McGinnis, W. Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila. PloS one. 8 (4), e61773 (2013).
- Evans, I. R., Zanet, J., Wood, W., Stramer, B. M. Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J. Vis. Exp. (36), e1696 (2010).
- Small, C., Paddibhatla, I., Rajwani, R., Govind, S. An introduction to parasitic wasps of Drosophila and the antiparasite immune response. J. Vis. Exp. (63), e3347 (2012).
- Repiso, A., Bergantiños, C., Corominas, M., Serras, F. Tissue repair and regeneration in Drosophila imaginal discs. Dev. Growth Differ. 53 (2), 177-185 (2011).
- Worley, M. I., Setiawan, L., Hariharan, I. K. Regeneration and transdetermination in Drosophila imaginal discs. Annu. Rev. Genet. 46, 289-310 (2012).

