Monitoring Electrical Signals from the Afferent Neurons in an Immobilized Zebrafish Larva
Abstract
Source: Lunsford, E. T., et al. Activity of Posterior Lateral Line Afferent Neurons during Swimming in Zebrafish. J. Vis. Exp. (2021).
This video demonstrates the recording of electrical signals from the afferent neurons in a paralyzed zebrafish larva by positioning a micropipette near the lateral line ganglion and forming a loose seal for signal capture. The extracellular solution maintains neuronal viability during this process.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of materials for electrophysiological recordings
- Make a silicone elastomer-bottomed recording dish.
- Dispense a thin layer of self-mixing silicone elastomer components (e.g., Sylgard) into a cover glass-bottomed tissue culture dish until it levels with the rim of a shallow well. Approximately 0.5 mL is sufficient.
- Cover and cure the dish for a minimum of 48 h at room temperature.
- Make dissection pins.
- Using a DC power supply, provide a negative charge (5 V) to a 100 mL beaker of etchant (3M KOH) and attach a tungsten wire (0.002 inch; 50.8 µm diameter) to the positively charged output.
CAUTION: Negative and positive wires should not contact one another during this procedure as you may run the risk of producing sparks, which could pose a potential fire hazard. - Etch the tungsten wire by quickly and repeatedly dipping the tip into the etchant bath until the tip narrows to a sharp point. Under a stereomicroscope, cut the wire approximately 1 mm from the tip with a straight-edge razor blade. Repeat three more times, and then insert pins into the cured recording dish using fine forceps.
- Using a DC power supply, provide a negative charge (5 V) to a 100 mL beaker of etchant (3M KOH) and attach a tungsten wire (0.002 inch; 50.8 µm diameter) to the positively charged output.
- Prepare recording electrodes.
- Using a horizontal micropipette puller with box filament, pull a borosilicate glass capillary tube (inner diameter: 0.86 mm, outer diameter: 1.50 mm) into electrodes with a 30 µm diameter tip with slight taper (Figure 1 Ai) that will be used to record afferent neurons from the posterior lateral line.
- Pull an additional borosilicate glass capillary tube into a pair of electrodes with smaller tip diameters (1-5 µm). Holding one electrode in each hand, gently run the tips across one another to break them to a ~30° angle. Using a microforge, polish the beveled tip until smooth.
- Generate the protocol in electrophysiology recording programs.
- Ensure the right head stage is connected to the Channel 1 input in the back of the microelectrode current and voltage clamp amplifier for the afferent neuron recordings.
NOTE: Computer specifications for the current and voltage clamp amplifier minimally require a 1 Ghz or better processor, Windows XP Pro or Mac OS X 10.46.6, a CD-ROM drive with 512 MB RAM, 500 MB hard drive space, and 2 USB ports. - Open the computer-controlled amplifier software.
- Set Channel 1 to current-clamp mode by clicking the IC button.
- Input the following parameter under the I-Clamp 1 tab. Primary Output: 100x AC Membrane Potential (100,000 mV/mV), Gain: 1,000, Bessel: 1 kHz, AC: 300 Hz, Scope: Bypass. Secondary Output: 100x AC Membrane Potential (100 mV/mV), Gain: 1, Lowpass Filter: 10 Hz.
- Save the channel parameter as Ch1_Aff.
- Install and open the patch clamp electrophysiology software.
- Click on Configure and select Lab Bench to set up the analog signals by adding Ch1_Aff to Digitizer Channels (e.g., Analog IN #0 and Analog IN #1) connected by BNC coaxial cable to the amplifier's corresponding Channel 1 Scaled Outputs. Click on OK.
NOTE: The minimum computer requirements for the digitizer are: a 1 Ghz or better processor, Windows XP Pro or Mac OS X 10.46.6, CD-ROM drive 512 MB RAM, 500 MB hard drive space, and 2 USB ports. - Click on Acquire and select New Protocol.
- In the Mode/Rate tab, select Gap Free; under Acquisition Mode, set Trial Length to either Use available disk space (i.e., record until stopped) or a desired set Duration (hh:mm:ss), and then set the Sampling rate per signal (Hz) to 20,000 to maximize the resolution.
- Under the Inputs tab, select the Analog IN Channels that were previously configured (in step 1.4.6) and select Ch1_Aff for the corresponding channel.
- Under the Outputs tab, select Cmd 0 and Cmd 1 for Channel #0 and Channel #1, respectively.
- Connect Channel 1 Command on the amplifier to Analog Output 0 on the digitizer via BNC coaxial cable.
- The remaining tabs can remain under default settings. Click on OK and save the protocol.
- Ensure the right head stage is connected to the Channel 1 input in the back of the microelectrode current and voltage clamp amplifier for the afferent neuron recordings.
2. Solution preparation
- Prepare Hank's solution: 137 mM NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO4; pH 7.3. Dilute to 10% Hank's solution by adding the appropriate volume of deionized water to the stock.
- Prepare the extracellular solution: 134 mM NaCl, 2.9 mM KCl, 1.2 mM MgCl2, 2.1 mM CaCl2, 10 mM glucose, and 10 mM HEPES buffer, pH 7.8 adjusted with NaOH. Vacuum filter the extracellular solution through a filter with a 0.22 µm pore size.
- Prepare α -bungarotoxin: Dissolve 1 mg of lyophilized α-bungarotoxin in 10 mL extracellular solution to produce 0.1% dilution.
- Prepare Euthanasia solution: 50% (mg/L) buffered pharmaceutical-grade MS-222 in 10% Hank's solution.
CAUTION: α-bungarotoxin is a potent neurotoxin that paralyzes muscles by blocking cholinergic receptors. Gloves are required and eye protection is recommended while handling the paralytic.
3. Preparation of larvae for electrophysiological recordings
- Immobilize zebrafish larvae.
- Use larvae from the laboratory-bred population of zebrafish (Danio rerio; 4-7 days post fertilization) and house in embryo solution (10% Hank's solution) at 27 °C.
- Using a large-tipped transfer pipette, transfer the larva from the housing into a small Petri dish (35 mm) and remove as much of the surrounding solution as possible.
NOTE: Removing the embryo solution prevents dilution of the paralytic and increases its efficacy. The corner of a task wipe can be used to wick away the remaining embryo solution, and it is critical not to contact the larvae or leave them exposed to air. - Immerse larvae in 10 µL of 0.1% α-bungarotoxin for approximately 5 min.
NOTE: The time necessary to immobilize larvae varies between preparations. A healthy preparation depends on closely monitoring sustained fast blood flow and decreased motor responses. Brief overexposure to the paralytic can lead to a slow decline in the preparation's overall health, even after a thorough washout. It is best to apply a wash before the larva is completely immobilized while it still shows signs of subtle muscle vibrations. - Wash the paralyzed larva with the extracellular solution and bathe for 10 min.
NOTE: The washout allows for the larva to transition from subtle muscle vibrations to complete paralysis. Also, α-bungarotoxin is an antagonist of the nicotinic acetylcholine receptor (nAChR) α9-subunit, which is a critical component of the endogenous feedback circuit this protocol observes; however, this effect has been shown to be reversible in Xenopus and zebrafish hair cells after a 10 min washout.
- Pin fish in the recording dish.
NOTE: Anesthesia (e.g., MS-222, Tricaine) is not required while preparing larval zebrafish (4-7 dpf) as it interferes with animal health. In fact, larval zebrafish are exempt from certain vertebrate protocols.- Using a transfer pipette, move the larva from the extracellular solution bath to the silicone-bottomed recording dish. Fill the remainder of the dish with the extracellular solution.
- Under a stereomicroscope, gently position the larvae with fine-tipped forceps above the center of the silicone mat, lateral side up, with the body's anterior and posterior ends running left to right, respectively. Then grasp an etched pin from the silicone mat using fine-tipped forceps and insert the pin, orthogonally to the silicone, through the dorsal notochord of the larvae directly dorsal to the anus. Insert the second pin through the notochord near the end of the tail and insert the third pin through the notochord dorsal of the gas bladder (Figure 1B).
NOTE: While inserting pins, it is important to target the center of the notochord width to prevent impinging blood flow or damaging surrounding musculature. The notochord is dorsal to the posterior lateral line nerve, so with proper pinning, no damage is expected to the lateral line sensory neurons. Insert after first contact so as to not disturb the surrounding tissue. Ideally, the diameter of the pin is less than half the width of the notochord to ensure clean insertion. If the pins exceed this width, repeat step 1.2 until the desired pin width is attained. If the blood flow slows after pinning, repeat from step 1.3 onward with a new specimen. - Insert the fourth pin through the otic vesicle while providing slight rotation towards the anterior as the pin inserts into the encapsulant (Figure 1B). As a slight rotation is applied, watch for the tissue between the cleithrum and otic vesicle to reveal the cluster of afferent somata.
NOTE: Angled insertion of the fourth pin is to ensure the exposure of the posterior lateral line afferent ganglion, which is otherwise obstructed by the large otic vesicle.
4. Afferent neuron recording
- Fill the afferent recording electrode with 30 µL of the extracellular solution, insert it into the right head stage pipette holder (Figure 1B, C), and lower it into the dish solution while applying positive pressure (1-2 mm Hg) produced by a pneumatic transducer.
- Locate and loosely attach to posterior lateral line afferent ganglion.
- Using a micromanipulator, lower the afferent electrode tip until it is holding position above the cleithrum.
- Increase the magnification to 40x immersion and locate the intersection of the posterior lateral line nerve and cleithrum. Follow the lateral line nerve anterior from the cleithrum to where the fibers innervate the posterior lateral line afferent ganglion, distinguishable by the discrete cluster of soma (Figure 1F).
- Bring the electrode tip over the afferent ganglion and lower the pipette until the tip contacts the epithelium. Gently, maneuver the electrode so that the entire tip circumference contacts the afferent ganglion.
- Apply negative pressure (20-50 mm Hg) with the pneumatic transducer and hold.
NOTE: The negative pressure applied to the afferent ganglion is gentle. Increasing negative pressure can improve signal-to-noise, but afferent neuron health declines under sustained aggressive suction, which decreases the probability of a successful recording.
- Record afferent neuron activity.
- The left head stage should be connected to the amplifier, which relays the amplified signal into a digitizer that outputs the said signal into the patch clamp electrophysiology software to be monitored on an adjacent computer.
- In pClamp10, click on the Play button on the toolbar to monitor afferent neurons.
- Ensure that the whole cell, loose patch recording of afferent neurons is achieved once spikes occur spontaneously, roughly every 100-200 ms (Figure 1E).
- Gradually, increase the afferent neuron recording electrode pressure back to atmospheric (0 mm Hg) and hold for the remainder of the recording.
Representative Results
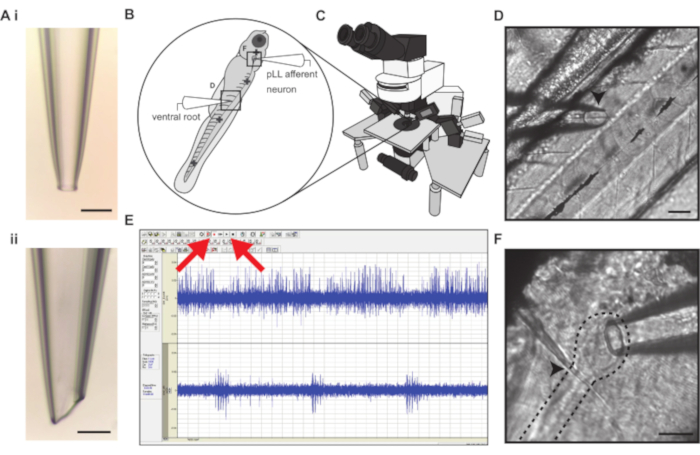
Figure 1: Simultaneous electrophysiological recording of posterior lateral line afferent neuron and ventral motor root activity. (A). Example of a loose-patch afferent (i) and ventral motor root (ii) recording electrodes. Scale bars represent 50 µm. (B) Larval zebrafish are paralyzed and pinned in four locations (cross symbols) to a Sylgard dish for recording stability. Bold crosses represent insertion points for pins. (C) The electrophysiology rig is mounted on a vibration-isolation table and consists of an upright fixed-stage microscope on a motorized controller capable of 40x magnification. Dual current clamp and voltage clamp head stages are mounted on micromanipulators. (D) The myomeres of the body musculature are separated by myosepta that serve as recording landmarks for motor neuron arborizations. The ventral motor root electrode approaches the ventral body (left) and is centered and lowered on top of a myoseptum (arrowhead). The scale bar represents 50 µm. (E) Screen capture of electrophysiological recording in real-time, allowing visualization of the spontaneous afferent activity (channel 1) and bursting ventral root activity indicative of fictive swim bout (channel 2). The Record and Play buttons are denoted with red arrows. (F) The posterior lateral line afferent ganglion (dashed line) lies just under the skin and can be identified by a tight cluster of afferent soma. The ganglion can be located by following the lateral line nerve past the cleithrum bone (arrowhead) to where it connects to the ganglion. The scale bar represents 30 µm.
開示
The authors have nothing to disclose.
Materials
| 100 mL beaker | PYREX | 1000 | Resceptacle for etchant |
| 10x water immersion objective | Olympus | UMPLFLN10xW | Low magnification for positioning larvae and recording electrode |
| 40x water immersion objective | Olympus | LUMPLFLN40XW | Higher magnification for position electrode tip and establishing patch-clamp |
| BNC coaxial cables | ThorLabs | 2249-C-12 | Connecting amplifier and digitizer channels; require 4 |
| Borosilicate glass capillaries w/ filament | Warner Instruments | G150F-3 | Inner diameter: 0.86, outer diameter: 1.50; capillary glass used to form recording electrodes |
| Computer | N/A | N/A | Any computer should work |
| DC Power Supply | Tenma | 72-420 | Used for electrically etching dissection pins |
| Electrophysiology digitizer | Axon Instruments, Molecular Devices | Axon DigiData 1440A | Enables acquisition of patch-clamp data |
| Filament | Sutter Instrument Company | FB255B | 2.5 mm box filament used in micropipette puller |
| Fine forceps | Fine Science Tools | Dumont #5 (0.05 x 0.02 mm) Item No. 11295-10 | Used to manipulate larvae and insert pins |
| Fixed stage DIC microscope | Olympus | BX51WI | Microscope used to visualize and establish patch-clamp recordings |
| Flexible, tapered pipette tip | Fisher Scientific | 02-707-169 | Flexible tips enable insertion into recording electrode to dispense extracellular solution at the tip |
| FluoroDish | World Precision Instruments Inc. | FD3510-100 | Cover glass bottomed dish recording dish |
| KimWipe | KimTech | 34155 | Task wipe used for wicking away excess fluid from larvae |
| Kwik-Gard | World Precision Instruments Inc. | 710172 | Self-mixing sylgard elastomer |
| Microelectrode amplifier | Axon Instruments, Molecular Devices | MultiClamp 700B | Patch clamp amplifier for dual channel recordings |
| Microforge | Narishige | MF-830 microforge | To polish recording electrode |
| Micromanipulator control unit | Siskiyou | MC1000-eR/T | 4-axis dial coordinator for controlling micromanipulator |
| Micropipette puller | Sutter Instrument Company | Flaming/Brown P-97 | For pulling capillary glass into recording electrodes |
| Microscope control unit | Siskiyou | MC1000e | Positions the microscope around the fixed stage and preparation |
| Motorized micromanipulator | Siskiyou | MX7600 | Positions the headstage and attached recording electrode for patch-clamp recording |
| MultiClamp Commander | Molecular Devices | 2.2.2 | Downloadable from Axon MultiClamp 700B Commander download page |
| Optical air table | Newport Corporation | VH3036W-OPT | Breadboard isolation table to float microscope and minimize vibrations during recordings |
| pCLAMP | Molecular Devices | 10.7.0 | Downloadable from Axon pCLAMP 10 Electrophysiology Data Acquisition & Analysis Software Download page |
| Petri-dish | Falcon | 35-3001 | Used to immerse larvae in paralytic |
| Pipette holder | Molecular Devices | 1-HL-U | Hold recording electrode and connect to the headstage |
| Pneumatic transducer | Fluke Biomedical Instruments | DPM1B | For controlling recording electrode internal pressure |
| Potassium hydroxide | Sigma-Aldrich | 221473-25G | Etchant for etching dissection pins |
| Silicone tubing | Tygon | 14-169-1A | Tubing to connect pneumatic transducer to pipette holder |
| Stereomicroscope | Carl Zeiss | Stemi 2000-C | Used to visualize pin tips and during preparation of larvae |
| Straight edge razor blade | Canopus | order from amazon.com | Cuts the tungsten wire while making dissection pins |
| syringe | Becton Dickinson Compoany | 309602 | Filled with extracellular solution to inject into recording electrodes |
| Transfer pipette | Sigma-Aldrich | Z135003-500EA | Single use, non-sterile pipette for transfering larvae |
| Tricaine methanesulfonate | Syndel | 12854 | Pharmaceutical aneasthetic used to euthanize larvae with high dosage. |
| Tungsten wire | World Precision Instruments Inc. | 715500 | 0.002 inch, 50.8 μm diameter; used to make dissection pins |
| Vacuum filtration unit | Sigma-Aldrich | SCGVU11RE | Single use, sterile, vacuum filtration units used to sterilize extracellular solution used for electrophysiology electrode ringer |
| Voltage-clamp current-clamp headstage | Molecular Devices | CV-7B | Supplied with MultiClamp 700B amplifier used as left and right headstages |
| α-bungarotoxin | ThermoFisher | B1601 | For immobilizing the larvae prior to recording |

