Generating an Organoid Culture from a Brain Tumor Cell Suspension
Abstract
Source: Sundar, S. J. et al. Maintaining Human Glioblastoma Cellular Diversity Ex vivo using Three-Dimensional Organoid Culture. J. Vis. Exp. (2022).
This video describes the preparation and cultivation of brain tumor organoids from single-cell suspension. Using a laminin-rich extracellular matrix as a scaffold and culture media for nutrients, cells are facilitated to multiply and self-organize within the organoid. Consequently, ex vivo organoids are generated, mimicking cellular diversity and the microenvironment of brain tumors.
Protocol
1. Making organoids from a single-cell suspension
- In an ice bucket or cold block, place the laminin-rich extracellular matrix (lrECM) and a small centrifuge tube. Place the appropriate amount of lrECM (16 µL x X number of intended organoids) into the small centrifuge tube.
- Create a mixture of cells in a total volume (4 µL x X number of intended organoids) containing 20,000 cells/organoid and add this to the small centrifuge tube with lrECM on ice.
- Carefully pipette 20 µL of the lrECM/cell suspension mixture onto parafilm molds, forming a pearl-like droplet.
- Be sure to thoroughly mix the lrECM/cell suspension mixture, as the cells tend to settle easily within the lrECM, resulting in heterogeneous organoids.
- Keep the lrECM/cell suspension mixture on ice. If lrECM warms, it can polymerize and compromise organoid formation.
- Be sure to cool the pipette tip every two to three organoids to prevent lrECM polymerization. Do not introduce air bubbles into organoids (avoid "double pushing" the pipette tip).
- Once the desired number of organoids are pipetted onto parafilm mold in a 10 cm culture plate, incubate at 37 °C for 1-2 h in a cell culture incubator.
- After organoids solidify, Neurobasal Media Complete (NBMc) media (Table 1) is used to flush them gently off the parafilm mold and into a new, sterile 10 cm culture plate with 20 mL of NBMc total. Using a Pipette-1000 (p1000) tip works best to flush organoids off the mold; they will slide off gently.
NOTE: About 15-20 organoids per 10 cm culture dish is recommended. - Place the 10 cm culture dish in an incubator (without shaking) for 4 days.
- After 4 days (Figure 1), exchange the media and place it on an orbital shaker at 80 RPM in the cell culture incubator. Exchange media every 2-3 days thereafter.
- Exchanging media with immature organoids is challenging because they are difficult to visualize. Tilt the cell culture dish and wait for at least 20 seconds; the organoids will settle at the bottom, and allow the media from above to be removed slowly with a large opening glass pipette.
- Pay careful attention to the collection of organoids at the bottom; if they appear stirred up with the force of media removal, pause and allow them to resettle. As organoids mature and are easier to visualize, this process becomes less nuanced.
NOTE: Sometimes, placing a piece of dark paper beneath the cell culture plate can help visualize immature organoids. Do not use a Pasteur pipette with vacuum suction to remove media, as it is very easy for the organoids to be sucked up and lost this way. - When organoids are first established, they do not consume media as rapidly as mature organoids. Begin with 50% media exchanges to reduce unnecessary media use and reduce the chance of accidentally damaging or aspirating new organoids during the media exchange process.
Table 1: Neurobasal medium complete (NBMc) formulation
| Component | Quantity |
| Neurobasal medium minus phenol red | 500 mL |
| B-27 supplement minus vitamin A (50x) | 10 mL |
| Antibiotic-antimycotic (100x) | 5 mL |
| Sodium pyruvate (100 mM) | 5 mL |
| Glutamine in 0.85% NaCl (200 mM) | 5 mL |
| Recombinant human FGF basic (250 µg/mL) | 20 µL |
| Recombinant human EFG protein (250 µg/mL) | 20 µL |
| Phenol red | 500 µL |
Representative Results
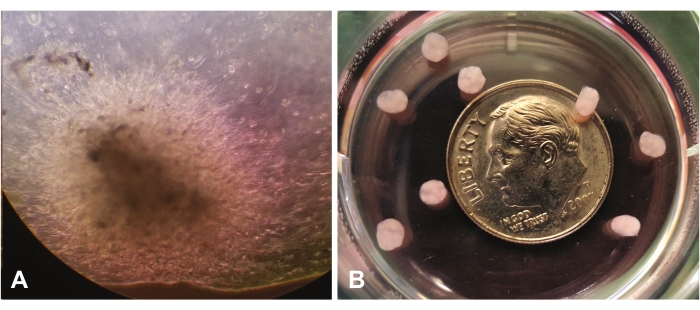
Figure 1 shows early organoid growth seen via light microscopy at 10x magnification. Figure 1A shows the migration and invasion of single cells through lrECM in the center view. The cells will continue to expand and 'colonize' the lrECM, and they will appear more dense and eventually opaque by visual inspection. Figure 1B shows several mature organoids (at 7 weeks) without magnification relative to the size of a dime.
開示
The authors have nothing to disclose.
Materials
| Antibiotic-antimycotic | ThermoFisher Scientific | 15240062 | Antibiotic-antimycotic |
| B-27 supplement minus vitamin A | ThermoFisher Scientific | 12587001 | B-27 supplement minus vitamin A (50x) |
| Matrigel | ThermoFisher Scientific | 354234 | Laminin-enriched extracellular matrix |
| Neurobasal media minus phenol red | ThermoFisher Scientific | 12349015 | Neurobasal media minus phenol red (500 mL) |
| Phenol red | Sigma | P0290 | Phenol red (0.5%) |
| Recombinant human EGF protein | R&D systems | 236-EG-01M | Recombinant human EGF protein (250 µg/mL) |
| Recombinant human FGF basic | R&D systems | 4144-TC-01 | Recombinant human FGF basic (250 µg/mL) |

