Establishing a Three-Dimensional Culture of Rat Brain-Derived Glial Cells
Abstract
Source: Koss, K. M, et al. Improved 3D Hydrogel Cultures of Primary Glial Cells for In Vitro Modelling of Neuroinflammation. J. Vis. Exp. (2017).
This video demonstrates a technique to establish a three-dimensional culture of glial cells. Rat-brain-derived glial cells are encapsulated within a photocrosslinkable polymer matrix containing extracellular matrix components. Upon incubating in a growth medium, the glial cells proliferate within the supporting matrix to create a three-dimensional culture.
Protocol
The protocol for the brain tissue extraction from day 1 Sprague Dawley rat pups, euthanized by decapitation, was approved by the Animal Care and Use Committee at the University of Alberta.
1. Microglia and Astrocyte Isolations
NOTE: All media for isolation and cell culture is preheated to 37 °C in a water bath. Hank's balanced salt solution (HBSS) has 1% penicillin-streptomycin (PS). All Dulbecco's modified Eagle's media with Ham's F12 nutrient mixture (DMEM/F12) is supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS). Only mixtures with trypsin lack FBS. All materials in this protocol are sterile (filter, alcohol, purchased, and autoclaved) and every step subsequent to step number 1.8 is performed in a biosafety cabinet with aseptic technique.
- Per brain, pre-heat 15 mL of HBSS and 12.5 mL DMEM/F12 to 37 °C in 50 mL conical centrifuge tubes, and approximately 2 mL of 0.25% trypsin with 1 mM ethylenediaminetetraacetic acid (EDTA) in a 15 mL conical centrifuge tube. Heat an additional 25 mL of DMEM/F12. Warm trypsin about 10 min prior to use to prevent loss of enzymatic activity.
- Pour enough warm HBSS into sterile, 6 and 10 cm culture dishes to cover the surface. Prepare one 10 cm dish for every two brains plus an additional 6 cm dish.
- Place up to 2 brains in each 10 cm2 dish (Figure 1A).
- Under a dissection microscope, use curved and straight forceps to separate the cortices along the midline, and the cerebellum with brain stem. This yields three sections of tissue per brain (Figure 1B).
- Peel the thin, semi-transparent, layer (meninges) of tissue containing blood vessels from each section of tissue and discard, transferring the remaining tissue into a separate culture dish. Grasp the thin layers exposed by the tissue cuts, with the forceps, and carefully pull until major portions are 'hanging off' the brain tissue. Finally remove this portion by gently tugging. See Figure 1C-1E for representative images of before, during, and after this step.
- In a biosafety cabinet, remove the remaining HBSS from the culture dish, carefully retaining the tissue. Macerate the tissue with a scalpel and transfer tissue to the pre-warmed 15 mL tube with trypsin.
- Incubate this tube for 25 min in a 37 °C bath to digest the tissue enzymatically.
- Centrifuge the tube down at 500 x g for 2 min at room temperature, and pour out the supernatant.
- Using a 10 mL serological pipette, add 10 mL of warm DMEM/F12 to inactivate the trypsin and triturate the solution 5 times to break up the tissue.
- Centrifuge the tube down at 500 x g for 2 min at room temperature, and pour out the supernatant.
- Using a 10 mL serological pipette, re-suspend pellet into 10 mL of warm DMEM/F12 and transfer the solution to a 50 mL Conical centrifuge tube.
- Using a 10 mL syringe with an 18 gauge needle, triturate the solution 3 times.
- Distribute this solution into increments of 12.5 mL of warm DMEM/F12 per brain.
- Pipette 1 mL increments of the triturated solution into a 12 well plate (1 per brain).
- Incubate at 37 °C and 5% CO2 in a humidified cell culture incubator. Replace media after three days, and refresh media every subsequent three days. After 2 weeks, the cells can be collected for experiments.
2. Macromer Synthesis
- Weigh 375 mg of hyaluronic acid (HA) salt in a 50 mL conical centrifuge tube and add 37.5 mL of distilled water.
- Flush the solution with a vortexer for 1 min and sonicate the mixture until it appears homogenous and loses its gel-like viscosity. This process may take 6 h.
- Transfer solution to a 100 mL beaker with a magnetic stir bar (38 mm), and stir vigorously at room temperature.
- Carefully titrate 5.0 M NaOH into the mixing HA and measure with pH paper until the pH stabilizes between 8 and 12.
- Collect 3 mL of fresh methacrylic anhydride (MA, 94%), blanketed by nitrogen during storage.
NOTE: MA is exposed to the atmosphere for long durations (days), it will lose its reactivity.
CAUTION: All use of MA should be done in a fume hood. - Add 200 µL of MA into the mixing HA solution. The reaction lowers the pH of the solution below 8.
- Repeat steps 2.4 to 2.6 until the 3 mL of MA have been added to the solution. This process may take up to 1-2 h.
- Allow the reaction to continue overnight at 4 °C, without mixing.
- Transfer solution to 400 mL of cold ethanol (EtOH) in a 500 mL beaker and allow precipitation for 24 – 72 h at 4 °C.
- Carefully decant the major portion of the solution into a separate beaker for disposal, and collect the precipitate in a 50 mL conical centrifuge tube. This may be distributed in 2-4 tubes, if necessary.
- Centrifuge the tubes at 200 x g in a centrifuge for 2 min at room temperature and dispose of the supernatant.
- Top up the tube with cold EtOH, mix the solution with a vortexer for 1 min, and repeat step 2.10-2.11.
- Add 25 mL of sterile deionized water, mix the solution with a vortexer for 1 min, sonicate (>20 kHz) for 1 h, and incubate at 4 °C overnight.
- Place the solution in a -80 °C freezer for 15 min or until frozen or flash freeze in liquid nitrogen.
CAUTION: When using liquid nitrogen, use protective gloves, a face shield, and an apron. - Freeze dry the tube (below 0.1 mBar and -30 °C) for 24 – 48 h until a snow-like powder is produced.
- At this point, test the sample for purity via 1H nuclear magnetic resonance, where the amount of methacrylate protons (peaks at 6.1 and 5.6 ppm), relative to methyl protons (1.9 ppm), on the HA backbone can be confirmed. A minimum ratio of 95% methacrylate to methyl protons is acceptable.
3. Gel Formation and 3D Encapsulation
- Coverslip and Mold Preparation
- Make a 50 mL distilled water solution of 2% 3-(Trimethoxysilyl) propyl methacrylate in a 50 mL conical centrifuge tube.
- Place 18 mm glass coverslips in solution and rock for 1 h at room temperature. Up to 50 coverslips can be added at once.
- Rinse coverslips by serially dipping each in 3 beakers of 100 mL deionized water.
- Dry the coverslips in a vacuum at 40 °C overnight with a desiccator and oven.
- Quickly immerse individual coverslips in 70% EtOH with forceps.
- Without drying, drop each coverslip into a well of a 12 well plate.
- Wash and aspirate each well with sterile deionized water (1 mL per well).
- Add 1 mL of sterile 2 µg /mL poly-L-lysine (PLL) to each well and incubate for >2 h.
- Aspirate PLL solution and allow the coverslips to air dry.
- Immerse polydimethyl siloxane (PDMS) molds (see Figure 2) in 70% EtOH, and place one upon the center of each coverslip, and allow to air dry and create a seal between the mold and glass.
- Prepare PDMS molds by casting PDMS premix reagents in a 10:1 ratio in a flat-bottomed polystyrene dish. The quantity of PDMS prepared is selected to yield a sheet approximately 1 mm thick. To form wells in the PDMS, cut the sheets with a circle punch with an inner diameter of 10.
- Cell Preparation
NOTE: All steps in the protocol are performed with sterile technique in a biosafety cabinet. The phosphate buffered saline (PBS) solution is adjusted to pH 7.4 for all steps.- 24 h prior to cell encapsulation in methacrylated hyaluronic acid (HAMA), refresh the medium for the 2-week primary cultures (from Step 1.15) in the 12 well plates. Add 1 mL of DMEM/F12 (10% FBS and 1% PS) medium per well.
- For each 12-well plate of cells prepare 12.5 mL of dilute trypsin (0.25% trypsin-EDTA diluted 30% with DMEM/F12 media), and 25 mL DMEM/F12 (10% FBS and 1% PS) and warm in at 37 °C in a water bath.
- Collect conditioned medium from plates using a 10 mL serological pipette (> 0.5 mL per well), thoroughly aspirate remaining liquid, and replace with 1 mL per well of dilute trypsin (0.075% trypsin-ethylenediaminetetraacetic acid [EDTA]) for 20-30 min in an incubator (37 °C, 5% CO2) until the confluent cell layer detaches from the plate.
- Recover suspended cell material with a 1 mL pipette (appears as a single floating piece) and collect in a 15 mL conical centrifuge tube. Dilute with an equivalent volume of DMEM/F12 (10% FBS and 1% PS), and centrifuge at 200 x g for 2 min at room temperature, discarding the supernatant.
- Resuspend the cell pellet in 10 mL warm DMEM/F12 (10% FBS and 1% PS), triturate the cells 5 times with a 10 mL pipette, and centrifuge at 200 x g for 2 min.
- Decant the supernatant, re-suspend the cells in 5 mL warm DMEM/F12, and transfer the cells to a 50 mL conical centrifuge tube.
- Using a 10 mL syringe with a 18 gauge needle, triturate the cell solution 3 times, and filter the suspension through a 40 µm cell sieve into a new conical centrifuge tube.
- Collect 10 µL of cell suspension, dilute 1:100 in warm DMEM/F12, and count cells with an automated cell counter.
- Incubate in a 37 °C water bath until encapsulation step.
- Cell Encapsulation
NOTE: All steps in the protocol are performed with sterile technique in a biosafety cabinet.- For each 12-well plate of 3D hydrogels warm 12.5 mL of DMEM/F12 and 12.5 mL of the conditioned DMEM/F12 media collected during cell preparation.
- Weigh the quantity of HAMA required for a final concentration of 0.5% wt/vol. Dissolve this HAMA in sterile filtered PBS at a concentration of (2% wt/vol); a concentration 4 times higher than the final concentration is utilized to accommodate the addition of cells and other reagents. One 12 well plate of hydrogels requires ~350 µL PBS with 7 mg HAMA.
- Sonicate (>20 kHz) solutions until HAMA is fully dissolved for 60 min.
- Prepare individual 10% solutions of both triethanolamine (TEA) and 1-vinyl-2-pyrrolidinone (NVP), and a 1 mM solution of Eosin Y (EY) in 1 mL aliquots of sterile PBS pH 7.4.
- For one 12 well plate, make a final 1.4 mL mixture of 1 x 107 cells, 0.5% wt/vol HAMA (350 µL of 2% wt/vol), 0.1% TEA (14 µL of 10%), 0.1% NVP (14 µL of 10%), and 0.01 mM EY (14 µL of 1 mM), and 20% basal lamina mixture (280 µL of stock). The remaining volume is PBS.
- Gently mix the solution and pipette 100 µL into each PDMS mold with a 1 mL tip.
- Expose samples to a high intensity green LED light (~520 nm, 60 mW, in an enclosed 20 cm x 20 cm x 20 cm box) for 5 min at room temperature.
- Add 1 mL per well of warm conditioned DMEM/F12 to a 12 well plate.
- Grasp each coverslip between thumb and forefinger, slowly peel the PDMS mold off with curved tweezers being careful not to displace the gel and place it into a well with conditioned medium.
- Add an additional 1 mL warm DMEM/F12 to each well.
- Incubate plates at 37 °C with 5% CO2 for 2 weeks, refreshing cell media every week by pipetting 1 mL of media from each well and replacing with 1 mL DMEM/F12.
Representative Results

Figure 1: Dissection of day 1 Sprague Dawley rat pup brain. Images of whole brain (A), dissection of cortices and cerebellum (B), a single cortex with meninges (C), peeling of the meninges with forceps (D), and the meninge-free cortex (E).
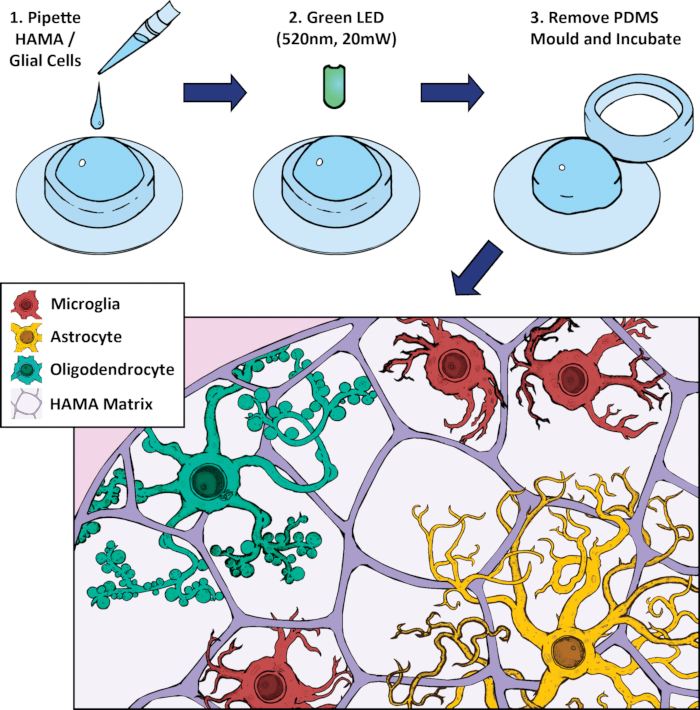
Figure 2: Schematic of methacrylated hyaluronic acid (HAMA)-based 3D cell culture and photopolymerization protocol. Glial cells (2-week primary rat brain culture) are mixed with HAMA and pipetted into a polydimethylsiloxane (PDMS) mold on a glass coverslip. This mixture is exposed to a high intensity green LED light to polymerize the hydrogel for 5 min. The mold is removed and the gel is incubated in media. Representative cells include microglia (red), astrocytes (yellow), and oligodendrocytes (green).
開示
The authors have nothing to disclose.
Materials
| 1. Materials for HAMA synthesis and photopolymerization | |||
| Hyaluronic acid (HA) | Sigma-Aldrich | 53747-10G | Streptococcus equi, MW: 1.5 – 1.8 X 10^6 |
| Methacrylic anhydride (MA) | Sigma-Aldrich | 275585-100ML | |
| Sodium hydroxide (NaOH) | Sigma-Aldrich | 221465-25G | |
| Ethanol (EtOH) | Commerical Alcohols Inc. | Anhydrous | |
| Phosphate buffered saline (pH 7.4) tablets | Fisher Scientific | 18912014 | |
| Triethanolamine (TEA) | Sigma-Aldrich | 90279-100ML | |
| 1-Vinyl-2pyrrolidinone (NVP) | Sigma-Aldrich | V3409-5G | |
| EosinY (EY) | Sigma-Aldrich | E6003-25G | |
| Polydimethylsiloxane (PDMS) Sylgard 184 Silicone Elastomer Kit | Dow Corning | ||
| 3-(Trimethoxysilyl)propyl methacrylate | Sigma-Aldrich | 440159-100ML | |
| Beaker (100 mL) | Corning | 1000-100 | |
| Beaker (500 mL) | Corning | 1000-600 | |
| pH paper (Labstick) | Sigma-Aldrich | 9580 | |
| 2. Materials for glial cell isolation and cell culture | |||
| P1-2 Sprague Dawley rat pups | Charles River | CD Sprague Dawley rat strain code 001 | |
| Dissector scissors – slim blades (small) | Fine Science Tools | 14081-09 | |
| Surgical scissors – Toughcut (large) | Fine Science Tools | 14130-17 | |
| Fine forceps (Dumont #5) | Fine Science Tools | 11521-10 | |
| Curved fine forceps (Dumont #7) | Fine Science Tools | 11271-30 | |
| Hank's balanced salt solution (HBSS) | Gibco | 14170-112 | |
| Dulbecco's modified Eagle's medium and Ham's nutrient mixture F-12 (DMEM/F12) | Gibco | 11320-033 | |
| Penicillin-streptomycin (PS) | Gibco | 15140-122 | |
| Fetal bovine serum (FBS) | Gibco | 12483-020 | |
| 0.25% Trypsin ethylenediaminetetraacetic acid (EDTA) | Gibco | 25200-072 | |
| Poly-L-lysine (PLL) | Sigma-Aldrich | P-6282 | |
| 50 mL conical centrifuge tube | Fisher Scientific | 05-539-13 | |
| 15 mL conical centrifuge tube | Fisher Scientific | 05-539-5 | |
| 12 well Tissue culture treated plates (Cellstar) | Greiner Bio-One | 665 108 | |
| 10 mL serological pipette | Fisher Scientific | 13-676-10F | |
| 25 mL serological pipette | Fisher Scientific | 12-676-10K | |
| Petri dish (60 mm X 15 mm) | Fisher Scientific | FB0875713A | |
| Petri dish (100 mm X 15 mm) | Fisher Scientific | FB0875712 | |
| Microscope Coverslip (18 mm) | Fisher Scientific | 12-545-100 18CIR |

