Using a Macrophage Conditioned Medium to Promote Neuron Extensions
Abstract
Source: Yun, H. J., et al. Neuron-Macrophage Co-cultures to Activate Macrophages Secreting Molecular Factors with Neurite Outgrowth Activity. J. Vis. Exp. (2018).
This video demonstrates an assay for promoting the growth of neuron projections known as neurite outgrowth using the macrophage-conditioned medium. Co-culture neurons and macrophages with cAMP and harvest the macrophage-derived conditioned medium. Growing the neurons with the conditioned medium promotes neurite outgrowths. These outgrowths are fluorescently labeled and visualized using a fluorescence microscope.
Protocol
1. Treatment of Db-Camp and Collection of Macrophage Conditioned Medium
NOTE: Start db-cAMP treatment 4 h after the neuron-macrophage co-cultures.
- Add 2 µL of 100 µM dibutyryl cyclic AMP (db-cAMP) solution to the neuron-macrophage co-cultures. Add the same volume of phosphate-buffered salin (PBS) for a control experiment.
- After 24 h, fill an empty well with 1 mL of macrophage culture medium in the same 6-well plate. Transfer the cell culture insert in the neuron-macrophage co-cultures to the empty well with macrophage culture medium. Keep the cells under the same condition for 72 h without changing the medium.
NOTE: We add only 1 mL of macrophage culture medium during conditioned medium (CM) collection to make concentrated CM. During the CM collection, if needed, we added more to completely cover the macrophages within the insert. - After 72 h, centrifuge the macrophage CM at 239 x g for 5 min to remove the cellular components. Pass the supernatant through a 0.2-µm filter to remove any remaining cellular debris. Store the collected CM at -70 °C until use.
2. Neurite Outgrowth Assay with Collected CM
- Before setting up a separate adult DRG neuron culture, pre-coat an 8-well chamber slide with poly-D-lysine and laminin. Incubate a 6-well plate with 0.01% poly-D-lysine at 37°C for 2 h or at 4°C overnight. Then, wash the plate twice with distilled water.
- Incubate the plate with laminin solution at a concentration of 3 µg/mL for 2 h at room temperature, and then wash the plate twice with distilled water. Dry the plate at room temperature at least for 1 h.
- Obtain dissociated adult DRG neurons in Neurobasal medium supplemented with B27. Plate 5 x 104 cells per well onto the pre-coated 8-well chamber slide.
- Place the chamber slide in a 37 °C incubator for 2 h, allowing the cells to attach to the bottom. Then, replace the culture medium with the thawed CM that is preheated at 37 °C.
NOTE: Be careful not to touch the bottom of the 8-well chamber slide when removing the culture medium and adding the collected CM. - 15 h after the initial plating, remove the medium and wash the cells with PBS once. Then, add 200 µL of ice-cold 4% paraformaldehyde solution to the wells, and incubate the cells with the paraformaldehyde solution for 20 min at 4 °C
NOTE: The DRG neuron culture for neurite outgrowth assay should be precisely restricted to 15 h. Under this condition, there is no appreciable neurite outgrowth. - Wash three times with ice-cold PBS and then perform blocking with 10% normal goat serum (NGS) with 0.1% Triton-X for 30 min. Incubate the fixed cells with primary antibody (anti-Tuj-1) solution diluted with 10% NGS (at a concentration of 1 µg/mL) for 4 h at room temperature or overnight at 4 °C.
- Wash three times with PBS, and then incubate the cells with secondary antibody (goat-anti mouse) solution for 2 h at room temperature. Then, wash twice with PBS and mount the culture slide with a coverslip (24 × 50 mm) using a mounting solution.
- Take images using a fluorescence microscope to visualize neurite outgrowth (Figure 1).
Representative Results
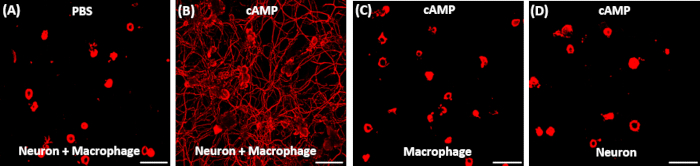
Figure 1: Representative results of neurite outgrowth assay using conditioned medium obtained from various conditions. (A, B) Adult DRG neurons were acutely dissociated and cultured for exactly 15 h. 2 h after plating, the culture medium was replaced with conditioned medium (CM) obtained from neuron-macrophage co-cultures treated with either PBS (A) or dibutyryl cAMP (db-cAMP) (B). Only the CM treated with db-cAMP exhibited robust neurite outgrowth activity. (C, D) CM obtained from cultures consisting of either macrophage (C) or neuron (D) alone that was treated with db-cAMP did not support neurite outgrowth. Scale bars indicate 200 µm.
開示
The authors have nothing to disclose.
Materials
| Cell culture insert transparent PET membrane 0.4μm pore size | Corning,Falcon | 353090 | Transparent PET membrane with 0.4-μm pore size, for 6-well plate |
| 70-μm nylon cell strainer | Corning, Falcon | 352350 | |
| 8-well culture slide | Biocoat | 354632 | with a uniform application of Poly-D-Lysine |
| Neurobasal medium | Thermo Fisher Scientific, Gibco | 21103-049 | Containing 1% glutamax and 1% penicilin-streptomycin |
| B-27 supplement, serum free | Thermo Fisher Scientific, Gibco | 17504-044 | extracellular solution |
| Glutamax | Thermo Fisher Scientific, Gibco | 35050-061 | |
| Penicillin-streptomycin | Thermo Fisher Scientific, Gibco | 15140-122 | |
| Poly-D-lysine Hydrobromide | Sigma-Aldrich | P6407-5MG | |
| Laminin | Thermo Fisher Scientific, Invitrogen | 23017-015 | |
| Adenosine 3', 5'-cyclic monophosphate, N6,O2'-dibutyryl-, sodium salt | Merck Millipore Corporation, Calbiochem | 28745 | |
| 10% Normal Goat Serum | Thermo Fisher Scientific | 16210072 | |
| Triton-X-100 | Daejung Chemical and Metal Co | 8566-4405 | |
| Anti β III tubulin (Tuj-1) | Promega Corporation | G7121 | Mouse monoclonal antibody |
| Goat anti-Mouse IgG (H+L) secondary antibody | Thermo Fisher Scientific, Invitrogen | A11005 | |
| Hemacytometer | Marienfeld-Superior | N/A | |
| Cell culture CO2 incubator | Panasonic | N/A | |
| Tabletop centrifuge | Sorvall | N/A | |
| Confocal microscope | Olympus America Inc | IX71 | |
| FBS (Fetal Bovine Serum) | VWR International, Hyclone | SH30919.03 |

