Generating Neuromuscular Junctions Using a Microfluidic Device
Abstract
Source: Stoklund Dittlau, K. et al., Generation of Human Motor Units with Functional Neuromuscular Junctions in Microfluidic Devices. J. Vis. Exp. (2021)
The video demonstrates the formation of neuromuscular junctions using a microfluidic device. Neural precursor cells and muscle progenitor cells are seeded into different wells within the device and left to mature into motor neurons and myotubes, respectively. The creation of a volume and chemical gradient leads to the development of active neuromuscular junctions.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
All reagents and equipment used in this protocol are listed in the Table of Materials and should be used sterile. Media should be heated to room temperature (RT) before use unless otherwise specified. For an overview of the co-culture protocol, please see Figure 1.
1. Differentiation of motor neuron progenitors from induced pluripotent stem cells (iPSCs)
- Follow the motor neuron differentiation protocol, adapted from a previous study, until reaching the day 10 neural progenitor (NPCs) state. According to the timeframe of the protocol, the differentiation is initiated on a Monday (day 0), which results in day 10 NPCs on a Thursday.
- Cryopreserve day 10 NPCs in knock-out serum replacement with 10% dimethyl sulfoxide (DMSO) at a density of 2 x 106– 4 x 106 cells per vial.
CAUTION: DMSO is toxic: handle in a fume hood with personal protective equipment.
NOTE: Approximately 50% of the day 10 NPCs are expected to be vital upon thawing. Stop the motor neuron differentiation protocol at this 'day 10 NPC' state and cryopreserve the NPCs to generate a large number of NPCs, which can be banked and used later, reducing the length of the overall timeline of the co-culture protocol from 28 days to 19 days total.
2. Derivation and maintenance of human mesoangioblast (MABs)
NOTE: MABs are vessel-associated mesenchymal stem cells, which in this case have been harvested from biopsies obtained from a 58-year-old healthy donor. Alternative commercial sources are available. The protocol to obtain MABs is briefly explained. For further information, refer to the detailed protocol. All MAB media should be heated to 37 °C before use.
- Mince the biopsy tissue and incubate on collagen (from calfskin) coated 6 cm dishes in a growth medium (Table 1) for 2 weeks. Change the medium every 4 days.
- To prepare collagen coating, dissolve 100 mg of collagen in 20 mL of 0.1 M Acetic acid. Collagen takes time to dissolve, so place the mixture on a rocking platform overnight at RT. The following day, top up with 80 mL of ddH2O to a final volume of 100 mL.
CAUTION: Acetic acid is toxic; handle in a fume hood with personal protective equipment.
NOTE: Collagen from calfskin coating can be reused up to 5x. Store at 4 °C. - Coat the entire surface of the dish or the flask with collagen, close and incubate for 20 min at RT inside a laminar flow. After 20 min, recover the collagen in a fresh container, close the empty dish/flask and leave for 10 min at RT in the laminar flow.
- Transfer the dish/flask to the incubator for overnight (or at least 6 h) incubation (37 °C, 5% CO2). Wash 5x with Dulbecco's phosphate-buffered saline without calcium or magnesium (DPBS) before plating cells.
- To prepare collagen coating, dissolve 100 mg of collagen in 20 mL of 0.1 M Acetic acid. Collagen takes time to dissolve, so place the mixture on a rocking platform overnight at RT. The following day, top up with 80 mL of ddH2O to a final volume of 100 mL.
- After 14 days, FACS (fluorescent activated cell sorting) sort the MABs for human alkaline phosphatase followed by further expansion. Maintain the MABs on collagen-coated T75 flasks in the growth medium and change the growth medium every 2 days (10 mL per flask).
- Cryopreserve, passage, or seed MABs in devices when reaching 70% confluence.
NOTE: MABs lose their myogenic potential due to spontaneous fusions upon cell-to-cell contact. Make sure not to exceed 70% confluence when expanding MABs. One 70% confluent T75 flask contains approximately 600,000-800,000 cells, which can be cryopreserved at 100,000 cells per vial. Each vial can later be thawed and seeded in a T75 flask for expansion. - To passage MABs, gently wash them once with 7 mL of DPBS and then incubate in 7 mL of MAB dissociation solution for 3 min at 37 °C in 5% CO2 to dissociate the cells.
- Neutralize the MAB dissociation solution with 7 mL of the growth medium, gently scrape the cells, and transfer the cell suspension to a 50 mL centrifuge tube. Gently wash the flask with an extra 5 mL of the growth medium to collect potentially remaining MABs.
- Centrifuge the cell suspension for 3 min at 300 x g, then passage directly to a new collagen-coated T75 flask for expansion, cryopreserve in knock-out serum replacement with 10% DMSO or count to seed in a microfluidic device.
NOTE: Passages are performed 1x-2x per week for cell expansion until a maximum passage number of 13. Upon dissociation, MABs appear spherical and large in shape when examined under the microscope.
3. Preparation of pre-assembled microfluidic devices – Day 9
NOTE: The protocol is adapted from the microfluidic device manufacturer's neuron device protocol and has been adjusted for the use of both pre-assembled and silicone devices. Here, pre-assembled devices are used for immunocytochemistry (ICC) and live-cell calcium transient recordings, while silicone devices are used for scanning electron microscopy (SEM). The timeline of the protocol follows the timeline for the motor neuron differentiation protocol.
- Prepare the microfluidic devices the day before seeding cells, as coating needs to incubate overnight. According to the motor neuron protocol, this will be a Wednesday. Add ~10 mL of 70%-100% ethanol to a 10 cm Petri dish. Use forceps to transfer the device from the shipping container to the Petri dish for sterilization.
- Submerge the device in ethanol for 10 s and transfer the device with forceps to a piece of paper to air dry in the laminar flow for ~30 min. Flip the device a few times to allow both sides to dry. When the device is dry, use forceps to move each device to an individual 10 cm Petri dish for easy handling.
CAUTION: Ethanol is toxic; handle in a fume hood with personal protective equipment - Coat the device with Poly-L-ornithine (PLO) (100 µg/mL) in DPBS and incubate at 37 °C, 5% CO2 for 3 h.
- Use a P200 pipette to add 100 µL of PLO in DPBS in a top well as close to the channel opening as possible and observe the fluid passing from the top well through the channel to the bottom well. Subsequently, add 100 µL of PLO in DPBS to the bottom well.
- Repeat on the other side of the microgrooves and finish by adding 100 µL on one side of the device to create a volume gradient between the two mirrored sides of the device to coat the microgrooves (e.g., right side 200 µL, left side 300 µL). After 3 h, wash the device 3x for 5 min with DPBS. Use a suction system if required.
NOTE: Make sure to avoid any air bubble formation in the channels at any point during the coating or culturing of the cells. Even small bubbles will expand over a short time, thereby inhibiting coating, cell seeding, or media flow across the channel. If the fluid stops in the channel during coating, resuspend the PLO solution directly into the channel from both sides. If bubbles are still present, use 200 µL of DPBS to flush the channel and repeat the coating process as stated above in steps 3.3.1-3.3.2. If bubbles appear after cell seeding, it is impossible to recover the device, as flushing the channel will damage the cells.
- Coat the device with laminin (20 µg/mL) in a Neurobasal medium and incubate overnight at 37 °C, 5% CO2. Follow the same instructions for PLO coating from steps 3.3.1-3.3.2.
- The following day, use a P200 pipette and position the tip in the well opposite to the channel opening to remove the laminin coating from the wells. Add DPBS to all the wells and leave the devices with DPBS in the laminar flow at RT for cell seeding.
NOTE: From this point on, it is important not to remove liquid (laminin coating, DPBS, media, fixation solution, etc.) directly from the channels, as this might cause air bubble formation. Always inspect the devices under the microscope before seeding cells.
4. Plating of NPCs in microfluidic devices – Day 10
NOTE: According to the motor neuron differentiation protocol, plating of day 10 NPCs occurs on a Thursday.
- Use freshly dissociated day 10 NPCs, or thaw 1-2 vials of banked NPCs per 10 mL of day 10 motor neuron medium (Table 2 and Table 3) with ROCK inhibitor (10 µL/mL) solution, and centrifuge the cell suspension at 100 x g for 4 min.
- Resuspend the cell pellet in 500-1000 µL of day 10 motor neuron medium with ROCK inhibitor (10 µL/mL) solution and count the live cells using any preferred counting method.
NOTE: As stated below, make sure to resuspend the NPCs into the correct amount of media to accommodate an optimal seeding volume. - Remove DPBS from two wells on one side of the microgrooves in the device with a P200 pipette and seed 250,000 NPCs per device in 60-100 µL of day 10 motor neuron media.
- In the top right well, seed 30-50 µL of the cell suspension (125,000 cells) close to the channel opening at a 45° angle and drag the remaining fluid gently along the well floor towards the center of the well with the pipette tip.
- Pause for a few seconds to allow the cell suspension to flow through the channel before repeating this in the lower well (125,000 cells in 30-50 µL). Use a pen to mark the seeded side "NPC" or equivalent for easy orientation of the device without a microscope.
- Incubate the device at 37 °C, 5% CO2 for 5 min to allow cell attachment before topping up the two-seeded wells with an additional day 10 motor neuron medium (total 200 µL/well) and incubate again at 37 °C, 5% CO2.
NOTE: Each well can contain 200 µL. Seeding cells in both wells and channels ensures a robust structure of the culture, lowering the risk of cell detachment during media changes. It is possible to seed fewer cells in just the channel. However, this will render the culture more susceptible to the volume current through the channels during each medium change.
- Use a P200 pipette to remove DPBS from the two wells on the other side of the microgrooves opposite the freshly seeded NPCs. Add 200 µL/well of day 10 motor neuron media and wait a few seconds between top and bottom well to allow media to flow through the channel. Then, add 6 mL of DPBS per 10 cm dish around the device to prevent evaporation of the medium during incubation.
NOTE: Add additional DPBS around the device during the culture period if needed. - Perform a full motor neuron medium change in both compartments of the device on day 11 (Friday), day 14 (Monday), and day 16 (Wednesday) (Table 2 and Table 3). Add fresh media supplements on the day of the medium change.
NOTE: From this point on, perform all medium changes with a P200 pipette. Always position the pipette tip away from the channel at the edge of the well and do not remove liquid directly from the channel. Be careful not to detach the silicone devices. Removing and adding medium should be done slowly to prevent cell detachment.- Carefully remove all media in both wells with NPCs by positioning the P200 pipette tip at the bottom edge of the well wall opposite the channel opening. Slowly add 50-100 µL of fresh motor neuron medium to the top well by positioning the P200 pipette tip at the top edge of the well wall opposite the channel opening.
- Pause for a few seconds to allow the medium to flow through the channel before adding 50-100 µL of motor neuron medium to the bottom well. Repeat this process carefully until both wells contain 200 µL/well. Repeat on the side without cells.
5. Plating of MAB in microfluidic devices – Day 17
- Approximately 7 days before seeding MABs in the microfluidic devices (day 10 of motor neuron differentiation), thaw MABs and seed them in the growth medium (Table 1) in a T75 flask coated with collagen to allow for sufficient cell expansion. See section 2.
- On day 17 of the motor neuron differentiation (Thursday), dissociate MABs as explained in step 2.4, resuspend the cell pellet in ~500 µL of growth medium and count the live cells using any preferred counting method.
NOTE: As stated below, make sure to resuspend the MABs into the correct amount of media to accommodate optimal seeding volume. - Remove the motor neuron medium on the unseeded side of the microgrooves in the device with a P200 pipette, wash gently with DPBS, and seed 200,000 MABs per device in 60-100 µL of growth medium.
- In the top right well, seed 30-50 µL of cell suspension (100,000 cells) close to the channel opening at a 45° angle and drag the remaining fluid gently along the well floor towards the center of the well with the pipette tip. Pause for a few seconds to allow the flow of cells through the channel before repeating in the lower well (100,000 cells in 30-50 µL).
- Incubate the device at 37 °C, 5% CO2 for 5 min to allow cell attachment before topping up the two freshly MAB-seeded wells with additional growth medium (total 200 µL/well). Incubate again at 37 °C, 5% CO2.
NOTE: No medium change is needed on day 17 on the motor neuron side of the device. Day 17 medium change according to the previously published motor neuron differentiation method is instead performed on day 18 (Friday).
6. Implementation of a volumetric and chemotactic gradient to promote the growth of motor neuron neurites towards the MAB compartment
- On day 18, perform a full medium change on the motor neuron side with day 18 motor neuron medium (200 µL/well). Follow the instructions for medium changes mentioned in steps 4.5.1-4.5.2. Initiate the MAB differentiation in the MAB compartment of the device (Table 2 and Table 4).
- Carefully wash the MAB compartments once with DPBS before adding preheated MAB differentiation medium (Table 4) supplemented with 0.01 µg/mL of human agrin (200 µL/well).
NOTE: MABs will fuse and form multinucleated myotubes over the time course of one week.
- Carefully wash the MAB compartments once with DPBS before adding preheated MAB differentiation medium (Table 4) supplemented with 0.01 µg/mL of human agrin (200 µL/well).
- On day 21, according to the motor neuron differentiation protocol (Monday), initiate the chemotactic and volumetric gradient (Table 2 and Table 3).
- Add 200 µL/well of motor neuron basal medium with 30 ng/mL of brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and ciliary neurotrophic factor (CNTF), human agrin (0.01 µg/mL), and laminin (20 µg/mL) to the myotube compartment (previously defined as the MAB compartment). Add motor neuron basal medium (100 µL/well) without growth factors to the motor neuron compartment.
Table 1: MAB growth medium. Medium can last 2 weeks at 4 °C. bFGF is added fresh on the day of use.
| Reagent | Stock concentration | Final concentration |
| IMDM | 1x | 80% |
| Fetal bovine serum | 15% | |
| Penicillin/Streptomycin | 5000 U/mL | 0.5% |
| L-glutamine | 50x | 1% |
| Sodium pyruvate | 100 mM | 1% |
| Non-essential amino acids | 100x | 1% |
| Insulin transferrin selenium | 100x | 1% |
| bFGF (added fresh) | 50 μg/mL | 5 ng/mL |
Table 2: Motor neuron basal medium. Medium can last 4 weeks at 4 °C.
| Reagent | Stock concentration | Final concentration |
| DMEM/F12 | 50% | |
| Neurobasal medium | 50% | |
| Penicillin/Streptomycin | 5000 U/mL | 1% |
| L-glutamine | 50x | 0.5 % |
| N-2 supplement | 100x | 1% |
| B-27 without vitamin A | 50x | 2% |
| β-mercaptoethanol | 50 mM | 0.1% |
| Ascorbic acid | 200 μM | 0.5 μM |
Table 3: Motor neuron medium supplements. Supplements are added fresh on the day of use to the motor neuron basal medium.
| Day | Reagent | Stock concentration | Final concentration | Compartment |
| Day 10/11 | Smoothened agonist | 10 mM | 500 nM | Both |
| Retinoic acid | 1 mM | 0.1 μM | ||
| DAPT | 100 mM | 10 μM | ||
| BDNF | 0.1 mg/mL | 10 ng/mL | ||
| GDNF | 0.1 mg/mL | 10 ng/mL | ||
| Day 14 | DAPT | 100 mM | 20 μM | Both |
| BDNF | 0.1 mg/mL | 10 ng/mL | ||
| GDNF | 0.1 mg/mL | 10 ng/mL | ||
| Day 16 | DAPT | 100 mM | 20 μM | Both |
| BDNF | 0.1 mg/mL | 10 ng/mL | ||
| GDNF | 0.1 mg/mL | 10 ng/mL | ||
| CNTF | 0.1 mg/mL | 10 ng/mL | ||
| Day 18 | BDNF | 0.1 mg/mL | 10 ng/mL | Motor neuron |
| GDNF | 0.1 mg/mL | 10 ng/mL | ||
| CNTF | 0.1 mg/mL | 10 ng/mL | ||
| Day 21+ | BDNF | 0.1 mg/mL | 30 ng/mL | Myotube |
| GDNF | 0.1 mg/mL | 30 ng/mL | ||
| CNTF | 0.1 mg/mL | 30 ng/mL | ||
| Agrin | 50 μg/mL | 0,01 μg/mL | ||
| Laminin | 1 mg/mL | 20 μg/mL | ||
| Day 21+ | No supplements | Motor neuron |
Table 4: MAB differentiation medium. Medium can last 2 weeks at 4 °C. Agrin is added fresh on the day of use.
| Day | Reagent | Stock concentration | Final concentration | Compartment |
| Day 18 | DMEM/F12 | 97% | MAB | |
| Sodium pyruvate | 100 mM | 1% | ||
| Horse serum | 2% | |||
| Agrin | 50 μg/mL | 0.01 μg/mL |
Representative Results
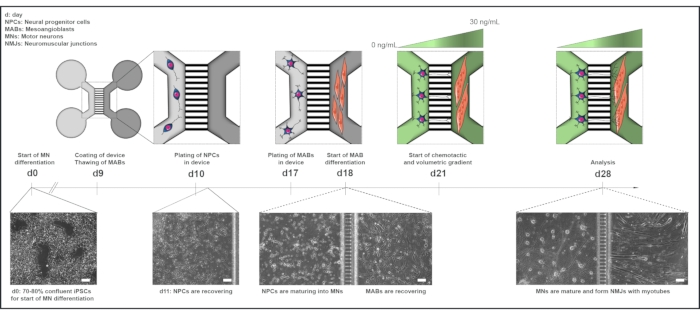
Figure 1: Schematic overview of the motor unit protocol in microfluidic devices. Differentiation timeline and co-culture overview from day 0 to day 28 according to the timeline of the motor neuron differentiation protocol. Motor neuron differentiation from iPSCs is initiated at day 0 and performed as stated previously for the following 10 days. On day 9, the device is sterilized and coated with PLO-laminin. MABs are thawed for expansion in T75 flasks. On day 10, the motor neuron-NPCs are plated in both wells and the channel of one compartment (light grey) of the device, where their differentiation into motor neurons is continued for a week. MABs are plated in both wells and the channel of the opposite compartment (dark grey) on day 17. On day 18, MABs differentiation into myotubes is begun. On day 21, a volumetric and chemotactic gradient is established to promote motor neuron-neurite polarization through the microgrooves of the device. The motor neuron compartment received 100 µL/well of motor neuron basal medium without growth factors (light green compartment), while the myotube compartment received 200 µL/well of motor neuron basal medium with 30 ng/mL of growth factors (dark green compartment) (Table 2 and Table 3). The culture is continued with the volumetric and chemotactic gradient for an additional 7 days until analysis on day 28. Bright-field images show cell morphology at day 0, day 11, day 18, and day 28 cultured in pre-assembled microfluidic devices. Scale bar, 100 µm.
開示
The authors have nothing to disclose.
Materials
| Acetic Acid | CHEM-Lab NV | CL00.0116.1000 | Coating component. H226, H314. P280 |
| Agrin (recombinant human protein) | R&D systems | 6624-AG-050 | Media supplement |
| Ascorbic acid | Sigma | A4403 | Media component |
| βIII-tubulin (Tubulin) | Abcam | ab7751 | Antibody (1:500) |
| β-mercaptoethanol | Thermo Fisher Scientific | 31350010 | Media component. H317. P280. |
| B-27 without vitamin A | Thermo Fisher Scientific | 12587-010 | Media component |
| BDNF (brain-derived neurotrophic factor) | Peprotech | 450-02B | Growth factor |
| bFGF (recombinant human basic fibroblast growth factor) | Peprotech | 100-18B | Growth factor |
| Collagen from calfskin | Thermo Fisher Scientific | 17104019 | Coating component |
| CNTF (ciliary neurotrophic factor) | Peprotech | 450-13B | Growth factor |
| DAPT | Tocris Bioscience | 2634 | Media supplement |
| DMEM/F12 | Thermo Fisher Scientific | 11330032 | Media component |
| DMSO | Sigma | D2650-100ML | Cryopreservation component. H315, H319, H335. P280. |
| Dulbecco's phosphate-buffered saline (DPBS) | Thermo Fisher Scientific | 14190250 | no calcium, no magnesium |
| Ethanol | VWR | 20.821.296 | Sterilization. H225. P280 |
| Fetal bovine serum | Thermo Fisher Scientific | 10270106 | Media component |
| Fluorescence Mounting Medium | Dako | S3023 | Immunocytochemistry component |
| GDNF (glial cell line-derived neurotrophic factor) | Peprotech | 450-10B | Growth factor |
| Horse serum | Thermo Fisher Scientific | 16050122 | Media component |
| IMDM | Thermo Fisher Scientific | 12440053 | Media component |
| Insulin transferrin selenium | Thermo Fisher Scientific | 41400045 | Media component |
| Knockout serum replacement | Thermo Fisher Scientific | 10828-028 | Cryopreservation component |
| Laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane | Sigma | L2020-1MG | Coating component and media supplement |
| L-glutamine | Thermo Fisher Scientific | 25030-024 | Media component |
| N-2 supplement | Thermo Fisher Scientific | 17502-048 | Media component |
| Neurobasal medium | Thermo Fisher Scientific | 21103049 | Coating and media component |
| Non-essential amino acids | Thermo Fisher Scientific | 11140050 | Media component |
| Parafilm M | Sigma | P7793-1EA | Storing equipment |
| Penicillin/Streptomycin (5000 U/mL) | Thermo Fisher Scientific | 15070063 | Media component |
| Petri dish (3 cm) | nunc | 153066 | Diameter: 3 cm |
| Petri dish (10 cm) | Sarstedt | 833.902 | Diameter: 10 cm |
| Plate (6-well) | Cellstar Greiner bio-one | 657160 | Culture plate |
| Poly-L-ornithine (PLO) | Sigma | P3655-100MG | Coating component |
| Retinoic acid | Sigma | R2625 | Media supplement. H302, H315, H360FD, H410. P280. |
| Revita cell supplement | Thermo Fisher Scientific | A2644501 | ROCK inhibitor solution |
| Smoothened agonist | Merch Millipore | 566660 | Media supplement |
| Sodium pyruvate | Life Technologies | 11360-070 | Media component |
| T75 flask | Sigma | CLS3276 | Culture plate |
| TrypLE express | Thermo Fisher Scientific | 12605010 | MAB dissociation solution |
| Tubocyrarine hydrochloride pentahydrate | Sigma | T2379-100G | Acetylcholine receptor blocker. H301. P280. |
| XonaChips pre-assembled microfluidic device | Xona Microfluidics | XC150 | Microgroove length: 150 μm |

