Generating Induced Pluripotent Stem Cell-Derived Cerebral Organoids
Abstract
Source: Koch, L. S., et al. Robust Tissue Fabrication for Long-Term Culture of iPSC-Derived Brain Organoids for Aging Research. J. Vis. Exp. (2023).
This video demonstrates an assay to generate cerebral organoids from induced pluripotent stem cells (iPSCs). Human iPSCs are induced to form embryoid bodies (EBs). The EBs are cultured over a polymer scaffold using an induction medium, promoting differentiation into neural stem cells, progenitor cells, and immature neurons. Finally, the EBs are embedded in a matrix and transferred to a maintenance medium for cerebral organoid formation.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
Figure 1 illustrates a schematic overview of the workflow of this protocol.
1. iPSC reprogramming and maintenance
- Collect 8 mL of the subject's blood (aged or disease patient; ages 65 and older) into cell preparation tubes (CPT) with sodium citrate or into ethylenediaminetetraacetic acid (EDTA) or heparinized tubes.
- Centrifuge at 1,800 x g for 30 min at room temperature (RT) to collect pellet containing only peripheral blood and no serum.
- Use specialized reprogramming vectors according to the manufacturer's protocol (see Table of Materials) to obtain iPSCs.
- Culture iPSCs in feeder-free, lactose dehydrogenase elevating virus (LDEV)-free reduced growth factor basement membrane matrix coated 6-well culture plates in Essential 8 (E8) media (see Table of Materials) at 37 °C in a humidified atmosphere with 5% CO2.
- Maintain iPSCs in E8 media for 3-4 days in order to avoid overcrowding or spontaneous differentiation.
- Validate the pluripotency of the iPSC lines using immunofluorescent markers (step 2) and test for mycoplasma (step 3).
2. Pluripotency staining
- To conserve solutions and antibodies, seed the cells on coated (step 1.4) 24-well culture plates 3-4 days prior to analysis. Aspirate the medium with a pipette and fix the cells with 4% formaldehyde in phosphate-buffered saline (1x PBS) for 15-20 minutes at RT.
- Wash the cells 3x with 1x PBS and permeabilize with 0.1% Triton-X 100 in 1% bovine serum albumin (BSA) in PBS for at least 15 min, but no more than 1 h at RT.
- Wash 3x with 1x PBS and block with 5% BSA in PBS for 30 min at RT.
- Wash again 3x with 1x PBS and add the antibodies Sox2 and Oct3/4 (1:100 dilution; see Table of Materials), and the nucleic stain DAPI in 1% BSA (1:4,000 dilution) overnight at 4 °C . Wrap in aluminum foil to protect from light.
- Wash 3x with 1x PBS and leave the cells in PBS. Image with a fluorescence microscope at a 10-20x magnification. Ensure that the pluripotency markers sex determining region Y-box 2 (Sox2) and octamer binding transcription factor 3/4 (Oct3/4) are localized in the nuclei of the cells.
3. Mycoplasma testing
NOTE: Refer to the detection kit's protocol (see Table of Materials) for detailed assay execution and analysis steps. The detection kit provides the reagent, the substrate, and the assay buffer for mycoplasma testing.
- Prior to passaging cells or refreshing medium, collect 2-3 mL of cell culture medium in a centrifuge tube and pellet any cells or debris at 200 x g for 5 min at RT. Store the supernatant at 4 °C for ≤5 days. Incubate the cells with the medium for at least 24 h to ensure a detectable signal.
- Add 100 µL of cell supernatant to a fresh tube or well of a white-walled 96-well plate (opaque bottom recommended, see Table of Materials). Reconstitute the reagent and substrate in the assay buffer and equilibrate for 15 min at RT.
- Add 100 µL of the reagent to the sample and incubate for 5 min at RT. Measure the luminescence with a luminometer (measurement #1).
- Add 100 µL of the substrate to the sample and incubate for 10 min at RT. Measure the luminescence (measurement #2).
- Determine the mycoplasma contamination by the ratio of measurement #2 to #1. Refer to the kit's manual for interpretation of the results.
4. Microfilament preparation
- Begin the preparation of the poly (lactic-co-glycolic acid) (PLGA, see Table of Materials) microfilaments by fraying the suture strand with the blunt end of a scalpel. Spay the frayed fiber lightly with 70% ethanol.
- Under the microscope and using a ruler, begin cutting the PLGA fiber into fragments of 500 µm to 1 mm long strands. Cut about 25 mm of the fiber in total. Keep the filaments in a 15 mL tube with a 1 mL antibiotic-antimycotic solution.
- In the hood, dilute the fiber solution with 10 mL of DMEM/F-12 (Table 1). Vortex well to mix the solution.
NOTE: Work in a cell culture flow hood in a sterile environment.
- In the hood, dilute the fiber solution with 10 mL of DMEM/F-12 (Table 1). Vortex well to mix the solution.
- Add 20 µL of the fiber solution to three embryoid body (EB) forming wells of a 96-well plate. Under brightfield microscopy, count and average the fibers per well. Dilute or concentrate to average 5-10 PLGA microfilaments per well. Prepare each well in this manner.
- The wells are now ready to be seeded with cells. Store the plate at room temperature until needed or at 4 ˚C for the following day.
5. Embryoid Body (EB) formation
NOTE: All media and solutions must be warmed to RT.
- Once the iPSCs have reached 70%-80% confluency (Figure 2A), they are ready to be passaged and used for EB formation. Check the cells with a microscope at 10x-20x magnification. Ensure that the colonies display minimal (<10%) areas of spontaneous differentiation.
- Aspirate the medium with a pipette and wash the cells once with Dulbecco's Phosphate-Buffered Saline (DPBS). Dissociate the colonies by adding 500 µL of cell detachment solution (see Table of Materials) or 0.5 mM of EDTA and incubate for 3-5 min at 37 °C.
NOTE: Work in a cell culture flow hood in a sterile environment. - Collect the released cells by adding 1 mL of fresh E8 media to each well and pipette gently until all cells are detached.
- Transfer 1.5 mL of cell suspension into a 15 mL tube, and add another 1 mL of fresh E8 media to reach a total volume of 2.5 mL.
- Centrifuge at 290 x g for 3 min at RT.
- Aspirate the supernatant with a pipette, resuspend the cell pellet in 1 mL of Essential 6 (E6, see Table of Materials) media supplemented with 50 µM Rho-associated protein kinase (ROCK) inhibitor, and count the cells using a hemocytometer.
- Prepare a cell suspension of 60,000-90,000 cells/mL, depending on the desired seeding density, in E6 media supplemented with 50 µM of ROCK inhibitor (see Table of Materials).
- Add 150 µL of the cell suspension into each well of a 96-well ultra-low attachment (ULA) plate (or a prepared concave plate). Seed 9,000-11,000 cells per well.
- Centrifuge the plate to force-aggregate the cells at 290 x g for 1 min at RT. Place the plate in the incubator at 37 °C in a humidified atmosphere with 5% CO2.
6. Neuroepithelial induction
- After 24 h, carefully aspirate 120 µL of the media with a pipette. Ensure not to aspirate the EB by lowering the pipette tip too far into the well.
- Add 150 µL of E6 media (at RT) supplemented with 2 µM of XAV939 and the Suppressor of Mothers against Decapentaplegic (SMAD) inhibitors: 10 µM of SB431542 and 500 nM of LDN 193189 per well (see Table of Materials).
- Change the medium daily with freshly prepared E6 media supplemented with 2 µM of XAV939, 10 µM of SB431542, and 500 nM of LDN 193189.
NOTE: By day 6 (DIV6), the EBs should have a diameter of 550-600 µm and be ready for further differentiation.
7. Organoid differentiation and maturation
NOTE: All media needs to be warmed to RT.
- At approximately DIV7, check whether all the EBs have reached a diameter of 550-600 µm and display a smooth and clear edge (Figure 2B); at this stage, they are ready to be embedded in an extracellular matrix (ECM).
NOTE: Work in a sterile environment. - Prepare dimpled embedding sheets from the thermoplastic sealing film (see Table of Materials) by placing a film sheet (about 4 in long) on an empty P200 box. Using a 15 mL conical tube or a 500 µL microcentrifuge tube, gently press down the film sheet into the holes to make 12 dimples. Spray the film sheet with 70% ethanol and let it dry inside the flow hood with the UV light switched on for at least 30 min.
- Thaw a sufficient quantity of basement membrane matrix (Matrigel, see Table of Materials) on ice and place it inside the flow hood.
NOTE: Per EB, approximately 30 µL of undiluted membrane matrix is needed. Always keep the membrane matrix below 4 °C to prevent it from gelling. Pre-chilled pipette tips are also recommended as they decelerate the matrix from polymerizing in the tip during pipetting, thereby reducing material loss. - Using a wide-bore P200 tip, transfer one EB to each dimple, and remove as much media as possible with a normal pipette tip. Be mindful not to let the EBs dry. Using a regular P200 tip, add ~30 µL of undiluted membrane matrix to each organoid, ensuring that the EB is at the center of the droplet.
- Once all EBs are embedded in the matrix, place the film sheet containing the EBs in a sterile Petri dish.
NOTE: Optionally, a small Petri dish filled with sterile water can be placed in the larger Petri dish next to the film sheet to prevent evaporation.- Transfer the dish to an incubator and incubate at 37 ˚C in a humidified atmosphere with 5% CO2 for approximately 10 min to let the membrane matrix solidify.
- For each set of 12 embedded organoids, prepare 5 mL of differentiation media with B27 without vitamin A (Table 1) in one well of a ULA 6-well plate. Pre-warm the plate to 37 °C in an incubator.
- Once the incubation time is finished, transfer the embedded EBs to the ULA 6-well plate by taking the film sheet and pushing out the dimples from the back of the sheet. If necessary, take 1 mL from the well and pipette it onto the sheet to help the droplets detach from the film.
NOTE: Important morphological changes can be seen 1 day after embedding; EBs go from having smooth edges to bulging protrusions forming buds (Figure 2C). - After 2 days (DIV9), perform a half-media change. Be careful not to aspirate or damage the membrane matrix droplets in the process.
- After 2 more days (DIV11), perform a full media change, supplementing the media with 3 µM of CHIR99021 (see Table of Materials).
- At DIV14, change the media to differentiation media with B27 with vitamin A (Table 1) for a gradual increase in organoid size.
- At DIV16, place the well-plate on an orbital shaker at 90 rpm inside an incubator. Change the media every 2 days.
- Every 40 DIV, dilute 500 µL of the membrane matrix for every 50 mL of media for additional nutrients in the media.
Table 1: Composition of the differentiation media used in the present study.
| Reagent | Final Concentration | Volume (50 mL total) |
| DMEM-F12 | 50% | 25 mL |
| Neurobasal Medium | 50% | 25 mL |
| N2 Supplement (100x) | 1x | 0.25 mL |
| B27 Supplement -/+ Vitamin A (50x) | 0.5x | 0.5 mL |
| Insulin | 0.25% | 12.5 µL |
| GlutaMAX (100x) | 1x | 0.5 mL |
| MEM-NEAA (100x) | 0.5x | 0.25 mL |
| HEPES (1 M) | 10 mM | 0.5 mL |
| Antibiotic/Antimycotic (100x) | 1x | 0.5 mL |
| 2-β-mercaptoethanol | 50 µM | 17.5 µL |
| NOTE: some DMEM-F12 already contains GlutaMAX, no need to add additional. | ||
Representative Results
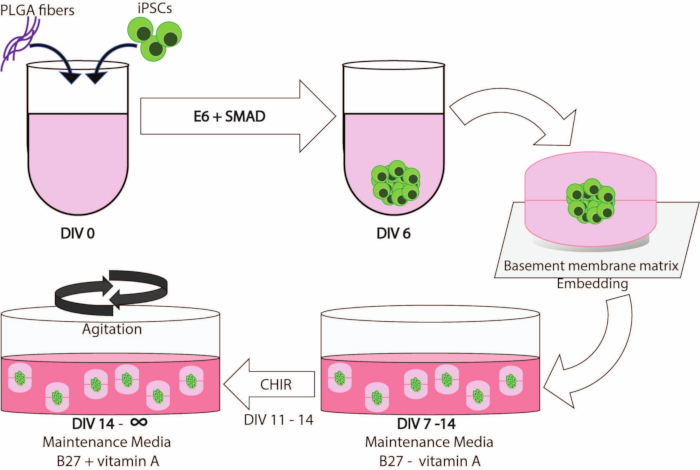
Figure 1: Schematic illustration of the workflow and timeline of this method.
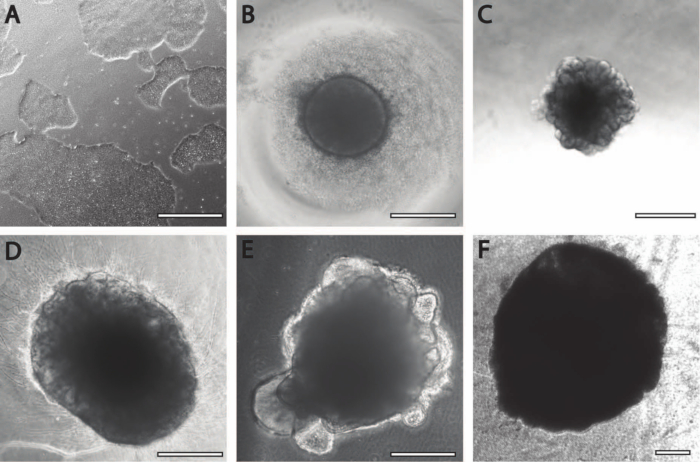
Figure 2: Differentiation and maturation of cerebral organoids. (A) Representative image of an iPSC culture at 70%-80% confluency. (B) Embryoid bodies (EBs) were generated and neuroepithelial formation was induced until DIV7. EBs were then embedded in the membrane matrix and further differentiated toward cerebral organoids. (C) Organoids at DIV10 displaying distinct budding formations. Organoids can be further matured in the membrane matrix using either (D) dimple or (E) sandwich embedding, here shown at DIV30. (F) Long-term culture shows cerebral organoids growing to significant sizes (DIV70). Scale bar = 500 µm.
開示
The authors have nothing to disclose.
Materials
| 2-β-Mercaptoethanol | Thermo Fisher Scientific | 31350010 | |
| 4′,6-Diamidino-2-phenylindoledihydrochloride (DAPI) | Invitrogen | D1306 | 67:40:00 |
| 6-well Clear Flat Bottom CELLSTAR Cell Culture Multiwell Plate | Greiner Bio-One | 657185 | |
| 6-well Clear Flat Bottom Ultra-Low Attachment Well Plate | Corning | 3471 | |
| 96-well Clear Round Bottom Ultra-Low Attachment Microplate | Corning | 7007 | |
| 96-Well, Nunclon Delta-Treated, Flat-Bottom Microplate | Thermo Fisher Scientific | 136101 | |
| Accutase | Sigma-Aldrich | 46964 | cell detachment solution |
| Antibiotic-Antimycotic (100x) | Gibco | 15240062 | |
| B27 Suppement (with Vitamin A) (50x) | Gibco | 17504044 | |
| B27 Supplement (minus Vitamin A) (50x) | Gibco | 12587010 | |
| BD Vacutainer™ Glass Mononuclear Cell Preparation (CPT) Tubes | Thermo Fisher Scientific | 02-685-125 | |
| Bovine Serum Albumin | Sigma-Aldrich | A9418 | |
| Centrifuge | Eppendorf | 5810 R | With plate holders |
| CHIR99021 | Selleck Chemicals | S2924 | |
| CytoTune Sendai Reprogramming Vector | Thermo Fisher Scientific | A1378001 | |
| ddPCR primers | human | MAPT | Bio-Rad | dHsaCPE192234 | |
| ddPCR primers | human | RBFOX3 (NeuN) | Bio-Rad | dHsaCPE5052108 | |
| DMEM/F12 | Thermo Fisher Scientific | 11320074 | |
| Doublecortin (DCX) | Santa Cruz Biotechnology | SC-8066 | 09:20 |
| Dulbecco's phosphate buffered saline (DPBS) | Thermo Fisher Scientific | 14190144 | no calcium, no magnesium |
| Eppendorf cups, 1.5 mL | Eppendorf | 0030 125.215 | |
| Essential 6 | Gibco | A1516401 | |
| Essential 8 | Gibco | A1517001 | |
| Ethylenediaminetetraacetic acid (EDTA) | Invitrogen | 15575020 | |
| Falcon tubes, 15 mL, conical | Greiner Bio-One | 188271-N | |
| Formaldehyde | Sigma-Aldrich | 252549 | 37% Stock solution, diluted to 4% in PBS |
| Geltrex LDEV-Free Reduced Growth Factor Basement Membrane Matrix | Thermo Fisher Scientific | A1413202 | |
| GlutaMax (100x) | Gibco | 35050038 | |
| Hemacytometer cell counter | Hausser scientific | 1490 | |
| HEPES Buffer | Thermo Fisher Scientific | 15-630-080 | |
| Insulin | Sigma-Aldrich | I9278 | |
| LDN 193189 | StemCell Technologies | 72147 | |
| MAP2 | Abcam | ab32454 | 04:20 |
| Matrigel Growth Factor Reduced (GFR) Basement Membrane Matrix, LDEV-free | Corning | 356230 | basement membrane matrix |
| MEM-Non Essential Amino Acid Solution (MEM-NEAA; 100x) | Thermo Fisher Scientific | 11140050 | |
| Multilabel Counter Victor 3 Plate Reader | Perkin Elmer | 1420 | luminometer |
| MycoAlert Mycoplasma Detection Kit | Lonza | LT07-318 | |
| N-2 Supplement (100x) | Thermo Fisher Scientific | 17502-048 | |
| NeuN | Millipore | MAB377 | 09:20 |
| Neurobasal Medium | Thermo Fisher Scientific | 21103049 | |
| Oct-3/4 Antibody (C-10) Alexa Fluor 647 | Santa Cruz Biotechnology | sc-5279 AF647 | 02:40 |
| Parafilm | Bemis | PM-996 | thermoplastic film sheet |
| PAX6 | Thermo Fisher Scientific | 42-6600 | 04:20 |
| Penicillin/Streptomycin | Gibco | 15070063 | |
| Poly(lactic-co-glycolic acid) (PLGA) microfilaments | Ethicon | J463 | |
| QX200 Droplet digital PCR system | Bio-Rad | 1864001 | |
| ROCK inhibitor (Y27632) | Selleck Chemicals | S1049 | |
| SB431542 | R&D Systems | 1614/50 | |
| SOX2 Monoclonal Antibody (Btjce), Alexa Fluor 488, eBioscience | Invitrogen | 53-9811-80 | 02:40 |
| Synapsin I (SYN) | Calbiochem | 574777 | 04:20 |
| Triton-X 100 | Sigma-Aldrich | T8787 | |
| TUJ1 | Santa Cruz Biotechnology | sc-80005 | Beta-3-tubulin; 1:500 |
| XAV939 | Tocris Bioscience | 3748 |

