Two-Dimensional Semi-Denaturing Agarose Gel Electrophoresis: A Technique to Separate Polymorphic Amyloids Fibers Based on Size Heterogeneity
Abstract
Source: Hanna-Addams, S., et al. Use of Two Dimensional Semi-denaturing Detergent Agarose Gel Electrophoresis to Confirm Size Heterogeneity of Amyloid or Amyloid-like Fibers. J. Vis. Exp. (2018).
This video demonstrates the two-dimensional semi-denaturing detergent agarose gel electrophoresis-based separation of amyloid fibers. The technique separates the polymorphic amyloid aggregates based on their heterogeneous size.
Protocol
1. Prepare Samples
- Culture 2 x 106 amyloid producing HT-29 colon cancer cells in a 10-cm tissue culture dish in 10 mL of Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum and penicillin-streptomycin. Culture cells overnight in a 37 °C incubator with 5% CO2.
- After the cells grow to 80% confluency, wash the cells with 10 mL of phosphate-buffered saline (PBS). Add 3 mL of Trypsin solution and incubate at 37 °C for 3 min.
- After the cells are totally dissociated from the dish, add 10 mL of culture medium and transfer the cells with a 10-mL pipette to a 15-mL conical tube. Centrifuge the cells at 1,000 x g for 3 min at room temperature. Aspirate the medium, resuspend the cells in 5 mL of culture medium, and count the cells using a cell counter. Plate 2 x 106 cells in each of two 10-cm dishes.
- Allow the cells to adhere and recover overnight in a 37 °C incubator with 5% CO2. Apply treatment to one dish to induce the formation of amyloids with 20 ng/mL Tumor Necrosis Factor-Alpha (TNF-α), 100 nM Smac-mimetic, and 20 µM pan-caspase inhibitor Z-VAD-FMK. The combination is abbreviated as TSZ. Treat the other dish with vehicle as a control.
- After the appropriate length of time, usually 6 h, harvest the cell lysate.
- Scrape the cells off the plate with a plastic scraper and use a 10-mL pipette to transfer into a 15-mL conical tube. Centrifuge the cells at 1,000 x g for 3 min at 4 °C.
- Wash the cells 2 times by resuspending in 10 mL of ice cold PBS and centrifuging at 1,000 x g for 3 min at 4 °C. Aspirate the PBS solution.
NOTE: The process can be paused here by freezing the cell pellet in liquid nitrogen and storing at ˗80 °C for up to 1 month. - Transfer the cell pellet to a 1.5-mL microcentrifuge tube and incubate in 0.3 mL of lysis buffer for 30 min on ice. Centrifuge at 20,000 x g for 15 min at 4 °C. The supernatant is the whole cell lysate.
NOTE: The process can be paused here by storing the sample at -20 °C for up to several months. - Measure the protein concentration by a Bradford assay. Add 4x SDD-AGE loading buffer to prepare 20 µL of 3 µg/µL sample and incubate at room temperature for 10 min.
2. Prepare and Run Gels
- Add 2 g of agarose to 200 mL of 1x Tris-acetate buffer (TAE) in a glass beaker and heat in a microwave to melt the agarose. Add 1 mL of 20% SDS for a final concentration of 0.1% SDS. Carefully swirl to mix. Take care not to generate bubbles after the SDS addition.
- Pour the agarose solution into a 15 cm x 14 cm gel slab. Use a 1-mL pipette to eliminate any bubbles. Place one 20-well comb at the top.
- First dimension: Pipette 60 µg of whole cell lysate in the far-right lane. Run the gel at 60 V for about 4 h (until the dye front is about ¾ through the gel) using the TAE containing 0.1% SDS as the running buffer.
- Second dimension: Carefully rotate the gel 90° counter-clockwise (Figure 1A). Run the gel at 60 V for about 4 h.
NOTE: The general running condition is 4 V/cm gel length. It is important that the running conditions are exactly the same for the first and second dimensions.
Representative Results
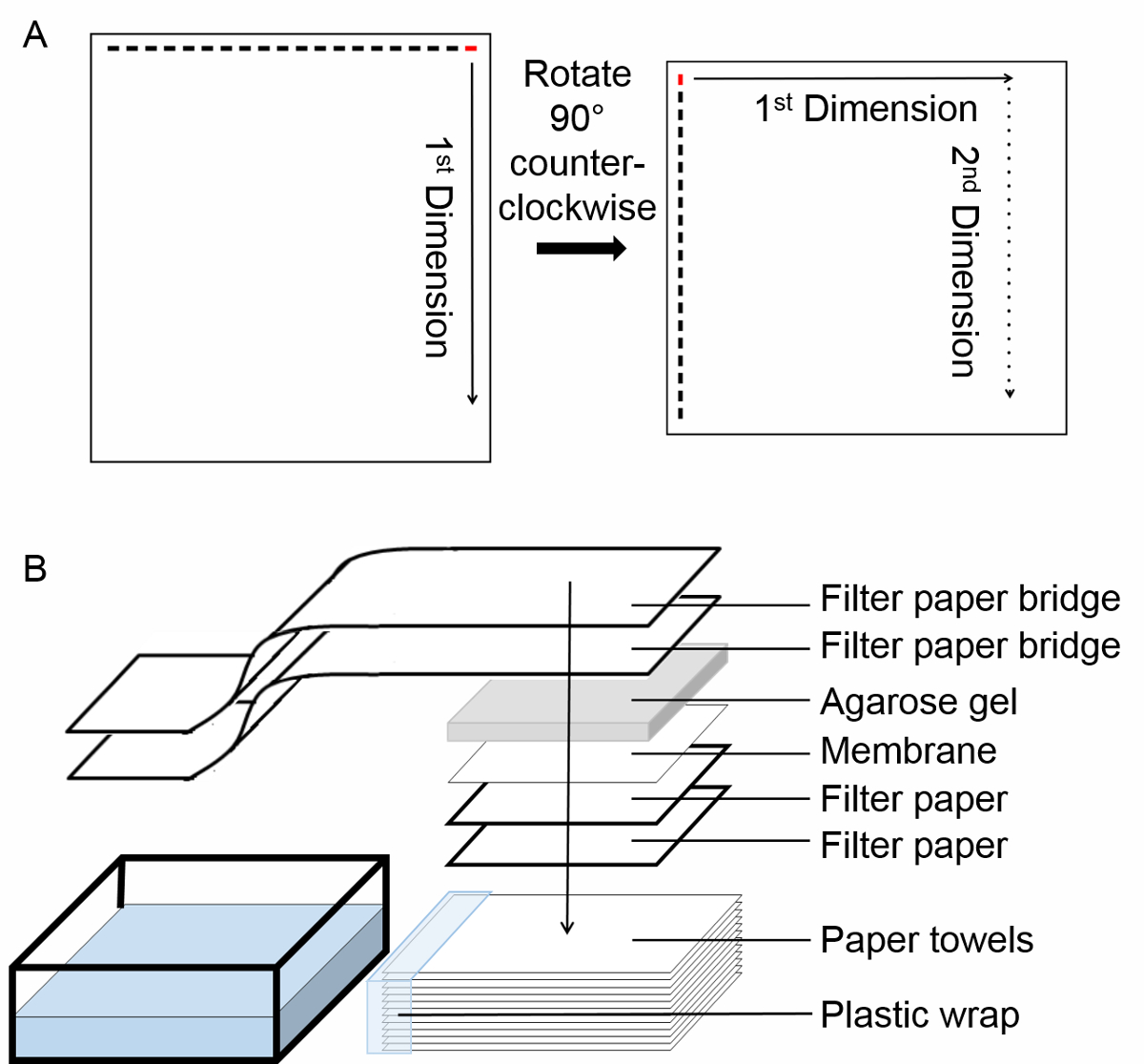
Figure 1. Experimental protocol. (A) Schematic of the two-dimensional semi-denaturing detergent agarose gel electrophoresis (2D SDD-AGE). Begin by loading the sample in the right most lane, labeled in red. After termination of the first dimension run, rotate the gel 90° counter-clockwise. Run the second dimension electrophoresis. Solid arrow indicates the direction of the first dimension run, dotted arrow indicates the direction of the second dimension run. (B) Schema of transfer by capillary action
開示
The authors have nothing to disclose.
Materials
| gel electrophoresis unit | Fisher | HE99XPRO | appratus for gel running. |
| agrose | VWR | 97062-250 | For agarose gel. |
| DMEM | Sigma | D6429 | for cell culture |
| fetal bovine serum | Sigma | F4135 | for cell culture |
| penicilin-streptomycin | Sigma | P4333 | for cell culture |
| Trypsin solution | Sigma | T4049 | for cell culture |
| PBS for tissue culture | Sigma | D8662 | for cell culture |
| recombinant TNF | made in our lab | for inducing necroptosis. See reference 11. | |
| smac-mimetic | gift from Dr. Xiaodong Wang | for inducing necroptosis. See reference 11. | |
| ZVAD-FMK | ApexBio | A1902 | for inducing necroptosis. See reference 11. |
| Cell Counter | Bio-Rad | 1450102 | Model TC20; for counting cells |
| Pierce™ BCA Protein Assay Kit | Thermo Scientific | 23225 | for measuring protein concentration in cell lysates |
| Cell lifter | Fisher | 07-200-364 | to remove cells from dish |
| Lysis Buffer (1 L) | 20 mL 1 M Tris pH 7.4 10 mL glycerol 30 mL 5 M NaCl 840 mL ddH2O 10 mL Triton-X100 (protease and phosphates inhibitors as desired) |
||
| 10X TAE (1 L) | 48.4 g Tris base 11.42 mL glacial acetic acid 20 mL 0.5M EDTA pH 8 ddH20 to 1 L |
||
| 4X SDD-AGE loading buffer (50 mL) | 5 mL 10X TAE 10 mL glycerol 4 mL 20% SDS 0.5 mL 10% bromophenol blue 31 mL ddH2O |
||
| PBST Wash Buffer (1 L) | 100 mL 10xPBS 800 mL ddH2O 1 mL Tween20 |
||
| 10X PBS (10 L) | 800 g NaCl 20 g KCl 144 g Na2HPO4·2H2O 24 g KH2PO4 add ddH2O to 10 L |

