11.16:
Ionic Crystal Structures
12,281 Views
•
•
Ionic crystals consist of two or more different kinds of ions that usually have different sizes. The packing of these ions into a crystal structure is more complex than the packing of metal atoms that are the same size.
Most monatomic ions behave as charged spheres, and their attraction for ions of opposite charge is the same in every direction. Consequently, stable structures for ionic compounds result (1) when ions of one charge are surrounded by as many ions as possible of the opposite charge and (2) when the cations and anions are in contact with each other. Structures are determined by two principal factors: the relative sizes of the ions and the ratio of the numbers of positive and negative ions in the compound. The size of the ion also depends upon the nature and magnitude of charge it possesses. As the positive charge increases on the cation it’s size decreases on contrary an increase in negative charge will increase the size of an anion, which inturn will affect the crystal structure.
In simple ionic structures, we usually find the anions, which are normally larger than the cations, arranged in a closest-packed array. (As seen previously, additional electrons attracted to the same nucleus make anions larger and fewer electrons attracted to the same nucleus make cations smaller when compared to the atoms from which they are formed.) The smaller cations commonly occupy one of two types of holes (or interstices) remaining between the anions. The smaller of the holes is found between three anions in one plane and one anion in an adjacent plane. The four anions surrounding this hole are arranged at the corners of a tetrahedron, so the hole is called a tetrahedral hole. The larger type of hole is found at the center of six anions (three in one layer and three in an adjacent layer) located at the corners of an octahedron; this is called an octahedral hole. Depending on the relative sizes of the cations and anions, the cations of an ionic compound may occupy tetrahedral or octahedral holes, Relatively small cations occupy tetrahedral holes, and larger cations occupy octahedral holes. If the cations are too large to fit into the octahedral holes, the anions may adopt a more open structure, such as a simple cubic array. The larger cations can then occupy the larger cubic holes made possible by the more open spacing.
There are two tetrahedral holes for each anion in either an hexagonal close packing (HCP) or cubic close packing (CCP) array of anions. A compound that crystallizes in a closest-packed array of anions with cations in the tetrahedral holes can have a maximum cation:anion ratio of 2:1; all of the tetrahedral holes are filled at this ratio. Examples include Li2O, Na2O, Li2S, and Na2S. Compounds with a ratio of less than 2:1 may also crystallize in a closest-packed array of anions with cations in the tetrahedral holes, if the ionic sizes fit. In these compounds, however, some of the tetrahedral holes remain vacant. The ratio of octahedral holes to anions in either an HCP or CCP structure is 1:1. Thus, compounds with cations in octahedral holes in a closest-packed array of anions can have a maximum cation:anion ratio of 1:1. In NiO, MnS, NaCl, and KH, for example, all of the octahedral holes are filled. Ratios of less than 1:1 are observed when some of the octahedral holes remain empty.
In a simple cubic array of anions, there is one cubic hole that can be occupied by a cation for each anion in the array. In CsCl, and in other compounds with the same structure, all of the cubic holes are occupied. Half of the cubic holes are occupied in SrH2, UO2, SrCl2, and CaF2. Different types of ionic compounds often crystallize in the same structure when the relative sizes of their ions and their stoichiometries (the two principal features that determine structure) are similar.
Examples of Ionic Crystal Structures
Caesium chloride (CsCl) is an ionic compound with a simple cubic lattice structure, where the cations and anions are of similar size. The chloride ions occupy the lattice sites and one caesium ion lies in the center of the unit cell (Figure 1). The coordination number for caesium chloride is 8, meaning each caesium ion is in direct contact with eight chloride ions (and vice versa). The caesium chloride unit cell contains one chloride anion and one caesium cation.
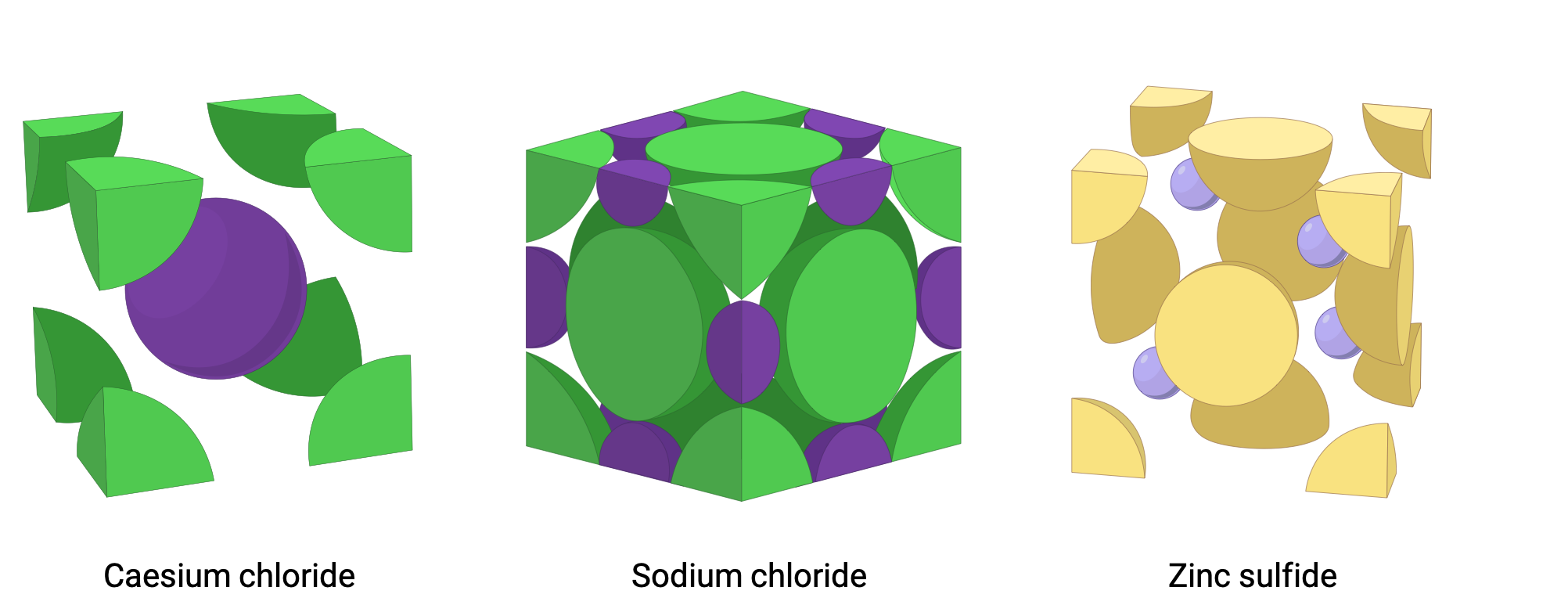
Figure 1. Unit cell structures of caesium chloride (simple cubic), sodium chloride (face centered cubic), and zinc sulfide (zinc blende).
Sodium chloride (NaCl) has a rock salt structure where chloride anions occupy the lattice sites of a face-centered cubic structure with the smaller sodium cations located in the spaces between the anions. NaCl has a coordination number of 6; each chloride anion is surrounded by six sodium cations and vice versa. A NaCl unit cell contains four chloride anions and four sodium cations.
Zinc sulfide (ZnS) has a zinc blende crystal structure with a coordination number of only 4. The sulfide anions occupy the lattice sites of a face-centered cubic structure with the smaller zinc cations occupying four of the eight tetrahedral shaped spaces located directly beneath each corner atom. Each ZnS unit cell contains four sulfide anions and four zinc cations. Other than zinc blende, ZnS can also be present in wurtzite structure, that exhibits hexagonal close packing unlike the cubic close packing of zinc blende. Similar to zinc blende both cation and anion have a coordination number of four and the cations occupy half of the tetrahedral voids (holes) whereas anion occupy the lattice sites of hexagonal structure.
Often crystal structures have an unequal number of cations and anions. Ionic compounds with a cation to anion ratio of 1:2 adopt the fluorite or the CaF2 structure. Sodium fluoride (NaF) is the simplest example with a structure similar to sodium chloride. CaF2 and MgF2 are other common examples.
Oxides such as TiO2 attain the crystal structure known as rutile. Here the coordination number of cations and anions are different. For example in the case of TiO2, the titanium cations will have a coordination number of six whereas the coordination number of oxygen anions will be three.
This text is adapted from Openstax, Chemistry 2e, Section 10.6: Lattice Structures in Crystalline Solids.
