11.9:
Clausius-Clapeyron Equation
49,175 Views
•
•
The equilibrium between a liquid and its vapor depends on the temperature of the system; a rise in temperature causes a corresponding rise in the vapor pressure of its liquid. The Clausius-Clapeyron equation gives the quantitative relation between a substance’s vapor pressure (P) and its temperature (T); it predicts the rate at which vapor pressure increases per unit increase in temperature.
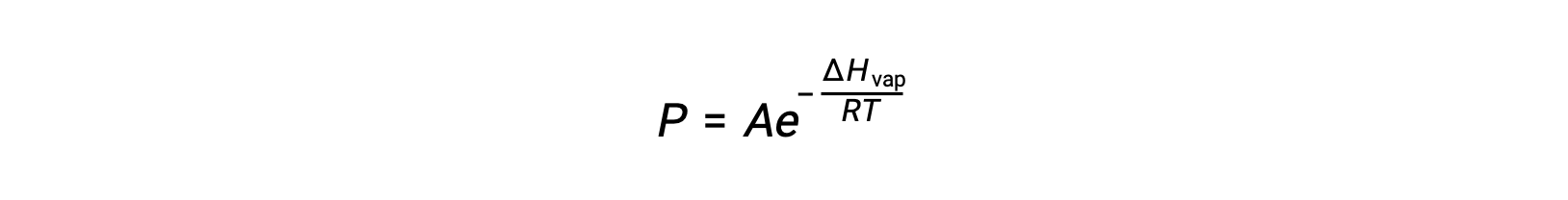
where ΔHvap is the enthalpy of vaporization for the liquid, R is the gas constant, and A is a constant whose value depends on the chemical identity of the substance. Temperature (T) must be in kelvin in this equation. However, since the relationship between vapor pressure and temperature is not linear, the equation is often rearranged into logarithmic form to yield the linear equation:
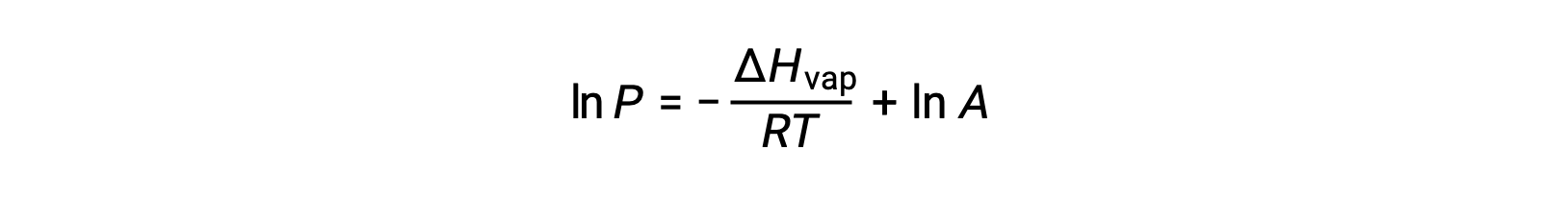
For any liquid, if the enthalpy of vaporization and vapor pressure at a particular temperature is known, the Clausius-Clapeyron equation allows to determine the liquid’s vapor pressure at a different temperature. To do this, the linear equation may be expressed in a two-point format. If at temperature T1, the vapor pressure is P1, and at temperature T2, the vapor pressure is P2, the corresponding linear equations are:
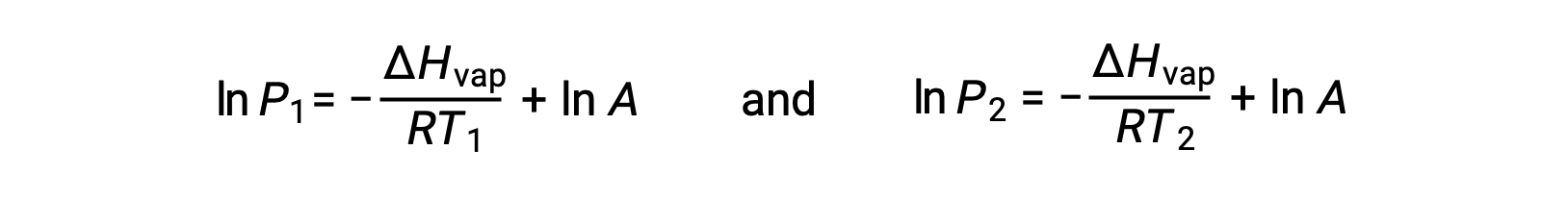
Since the constant, A, is the same, these two equations may be rearranged to isolate ln A and then set them equal to one another:
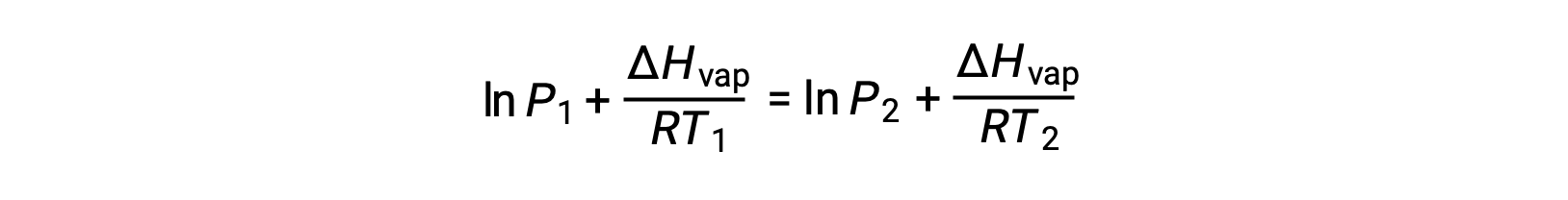
which can be combined into:
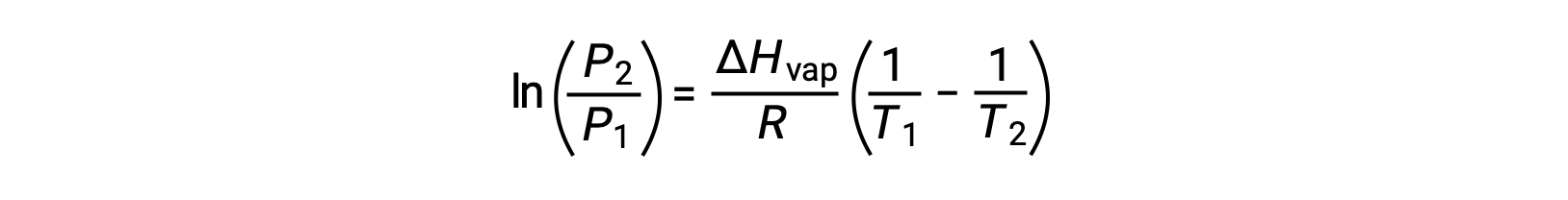
This text is adapted from Openstax, Chemistry 2e, Section 10.3: Phase Transitions.
