Demonstration of Membrane Transport of Histidine using Goat Intestinal Inverted Sacs: An Experiential Pedagogical Tool for Undergraduates
Summary
Here, we report an inexpensive and reproducible method demonstrating the membrane transport of histidine in a goat intestine. This process occurs by co-transporting histidine and sodium ions enabled by the sodium gradient across the enterocyte membrane. This method exploits experiential learning pedagogy to better understand solute movement across biological membranes.
Abstract
Histidine is an essential amino acid that is also a precursor for metabolites implicated in the immune system, pulmonary ventilation, and vascular circulation. Absorption of dietary histidine relies largely on the sodium-coupled neutral amino acid transport by the Broad neutral amino acid transporter (B0AT) present on the apical membrane of the enterocyte. Here, we demonstrate the absorption of histidine by the intestinal villus enterocytes from the lumen using goat jejunal inverted sacs. The jejunal sacs exposed to varying concentrations of sodium and histidine were assayed to determine the concentration of histidine inside the sacs as a function of time. The results show active histidine absorption. Increasing the concentration of salt resulted in higher absorption of histidine, suggesting a symport of sodium and histidine absorption in goat intestinal inverted sacs. This protocol may be applied to visualize the intestinal mobility of amino acids or other metabolites with appropriate modifications. We propose this experiment as an experiential pedagogical tool that can help undergraduate students comprehend the concept of membrane transport.
Introduction
Biological cells are surrounded by a membrane lipid bilayer that separates the intracellular cytosol from the extracellular content. The membrane serves as a semipermeable barrier that regulates the movement of solutes1. Transport across biological membranes is affected by a solute's permeability coefficient, which depends on several factors, including the concentration and charge of the solute. In general, solutes move through the membrane using three mechanisms (Figure 1): Passive diffusion, facilitated diffusion, and active transport2. Simple diffusion is the process by which soluble, uncharged, and non-polar solutes pass down their concentration gradient through a semipermeable membrane (Figure 1A). Membrane proteins do not assist in this process since it involves the movement of solutes from a region of higher concentration to a region of lower concentration. The rate of diffusion is based on Fick's Law3. On the other hand, facilitated diffusion is a protein-dependent transport wherein the membrane allows only selective solutes to pass along a concentration gradient without the expenditure of energy (Figure 1B). This kind of transport is specific and differs from simple diffusion in exhibiting saturation kinetics.
Active transport is a protein-dependent transport of molecules against their concentration gradient, i.e., from the region of lower concentration to a region of higher concentration with the use of ATP or ion gradients (Figure 1C). When the transporter hydrolyses ATP, the transport is termed primary active transport (Figure 1C; left panel). Another form of active transport is secondary active transport (Figure 1C; right panel). In secondary active transport, solutes are moved based on an electrochemical gradient. It takes place when a transporter protein couples the movement of an ion (typically Na+) down its concentration gradient with the movement of another molecule or an ion against its concentration gradient. This kind of solute movement can be a co-transport (Symport) wherein both the solute and the ion move in the same direction or an exchange (Antiport), in which case the solute and the ion move in opposite directions.
The dietary amino acids and monosaccharides from food sources are absorbed in the small intestine. The small intestine can be functionally divided into three segments: Duodenum, Jejunum, and Ileum (Figure 2). Absorption of solute occurs throughout the small intestine, with maximum absorption occurring at the jejunum and at the proximal end of the ileum. The intestinal enterocytes are polarized cells, and the tight junctions connecting two adjacent cells create two distinct membrane sites – the basolateral and the apical membrane site (Figure 2). The absorption of luminal solutes generated by digestion occurs on the apical membrane site4.
Histidine transport in the intestinal enterocytes at the apical membrane is an example of a secondary active symport that is sodium-dependent. At the basolateral end, the histidine entering the enterocyte moves down the concentration gradient into the hepatic portal circulation. The intracellular concentrations of sodium within the enterocyte are maintained at 12 mmoles/L1, which is lower than the extracellular/luminal concentrations, due to the active pumping of sodium out of the cell by Na+ K+ATPase located on the basolateral membrane (Figure 3). At the apical membrane of enterocytes, the B0AT and the sodium neutral amino acid transporter (SNAT) 5 are the major transporters that not only transport histidine but also amino acids such as asparagine and glutamine in a sodium-dependent cotransport5,6. Another transport protein called Large Amino acid Transporter (LAT)1 present at the basolateral membrane of enterocytes transports large neutral amino acids such as leucine, tryptophan, tyrosine, and phenylalanine across the membrane7.
With the objective of teaching the concept of membrane transport integrated with techniques such as spectrophotometry and routine biochemical assays through experiential learning pedagogy, it is imperative to develop methodologies that can not only demonstrate the concept in easy-to-understand terms but also enable participatory learning for undergraduate students. Currently, there are limited resources available to the students for hands-on engagement to learn such concepts of biochemistry. Here, we report a simple protocol for demonstrating Histidine transport across goat intestinal membrane that is easy to reproduce in undergraduate laboratories and can be adapted for evaluating the transport of other metabolites as well. More importantly, the method utilizes inexpensive materials in an undergraduate laboratory, thereby enabling experiential learning in even the simplest of laboratory settings.
Protocol
The entire protocol with all steps is depicted as a schematic diagram in Figure 4. The method is adapted from a previous study using rat intestines8. The experiment was performed in compliance with institutional guidelines. The samples used in this study were procured from a commercial vendor.
CAUTION: Wear gloves during this experiment.
1. Preparation of inverted Jejunum sacs
- Pre-treat and clean goat jejunum.
- Procure the entrails of a freshly sacrificed healthy goat from a licensed vendor.
- Place the entrails in enough 1x phosphate buffer saline (PBS) so that they are immersed completely for cleaning.
- Separate the small intestine from the large intestine, and make sure that the external connective tissue is removed using scissors.
- Gently flush the small intestine with 1x PBS using a 10 mL syringe, ensuring the removal of undigested food material to obtain a hollow bag of small intestine. Repeat this process at least thrice for effective cleaning.
NOTE: Avoid using excessive force or needles while flushing. The use of force can result in the puncturing of the intestinal walls and thus damage the intestinal enterocytes. - Measure the complete length of the small intestine using a ruler to obtain the three parts: Duodenum (1/4th of the small intestine) and the remainder split evenly into Jejunum and Ileum.
- Proceed with the experiment using the Jejunum part.
- Prepare inverted Jejunum.
- Clean the jejunum part by removing all the connective tissue using a blade.
- Cut the jejunum into 12-15 cm long portions, wash with 1x PBS, and place in a Petri dish containing PBS.
- Invert the jejunum portions using a glass rod to allow the villi portion to be exposed on the outside.
CAUTION: Avoid using a glass rod with sharp edges. Moreover, the diameter of the glass rod should not be greater than the diameter of the sac.
NOTE: At this point, one can visualize the villi under a light microscope (Figure 4). - Gently flush the inverted jejunum portions with 1x PBS.
- Check for any leaks from the sides of the inverted jejunum.
- Cut the inverted jejunum portions into 5 cm long sacs.
- Tie one end with twine and recheck for visual leaks at the tied end by filling the sacs with PBS.
- Tie the other end to create an empty inverted sac with villi on the external surface.
- Recheck for external leaks by patting the inverted sac on filter paper.
- Equilibrate the inverted sacs in 1x PBS.
- Proceed with the experiment on the same day or store the sacs overnight in 1x PBS at 4 °C.
2. Experimental setup for Histidine transport
- Assemble two sets of three 50 mL tubes according to different concentrations of sodium chloride as given in Table 1.
- Dip the prepared intestinal sacs in the specified tubes for 30 min and 60 min for each of the sets.
- After the designated time, empty the solution inside the sac in a separate 1.5 mL microcentrifuge tube and determine the volume.
- Aspirate the liquid inside the sac using a 2 mL syringe. Alternatively, untie one end of the sac and collect the contents in a fresh 1.5 mL tube.
- Assay 0.1 mL of the collected samples for histidine estimation and calculate the concentration using the standard graph.
3. Estimation of Histidine using Pauly's reaction
- Prepare a standard curve for histidine.
- Prepare solutions with varying concentrations of histidine as indicated in Table 2 using stock solutions of histidine (5 mM) and water.
- Add sulphanilic acid (0.5 mL) and sodium nitrate (0.5 mL) to each tube.
- Incubate the tubes for 5 min at room temperature, then add 1 mL each of sodium carbonate and ethanol to each tube.
- Incubate the tubes for 20 min at room temperature (~20 °C) and note the absorbance at wavelength 490 nm.
- Plot a standard curve of absorbance versus Histidine concentration.
NOTE: This test for histidine is based on the property of histidine to form a diazo compound (Figure 5).
Representative Results
The experimental workflow for demonstrating intestinal mobility of histidine through absorption of histidine by intestinal villi into the lumen of the inverted sacs is illustrated in Figure 4, Table 1 and Table 2. Three independent experimental setups were performed, and representative data are presented in Figure 6.
Under the given experimental conditions, Histidine estimation using Pauly's reaction followed Lambert Beer's law till 300 µM (Figure 6A). This test for histidine relies on the coupling between amino acids and the diazonium ion in the reagent. Pauly's reagent has sulphanilic acid dissolved in concentrated hydrochloric acid. This acid undergoes diazotization in the presence of sodium nitrate and HCl. The diazonium salt p-phenyldiazosulphonate reacts with histidine molecules under alkaline conditions to form a dark red/orange to a yellow colored complex, which can be spectrophotometrically read at 490 nm (Figure 5)9. It may be noted that this test is given by histidine and tyrosine. The test used in this study is a slightly modified version of the original reaction such that sodium nitrate is used directly at room temperature (~20 °C) rather than using sodium nitrite under chilled conditions9.
The positive Pauly's reaction observed with the sample recovered from inside the inverted sacs illustrates the absorption of histidine across the intestinal villus. Figure 6B shows a correlation between the uptake of histidine and sodium concentrations in jejunal enterocytes. An increase in the absorption of histidine as incubation time increases from 30 to 60 min is indicative of secondary active transport.
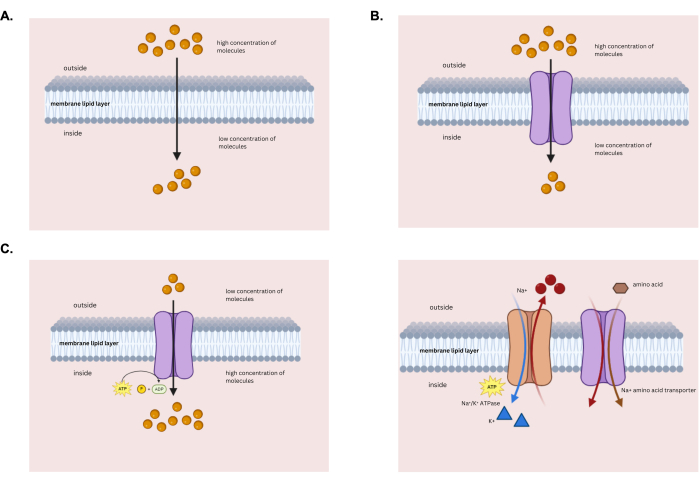
Figure 1: Different types of membrane transport. Schematic showing the various kinds of membrane transport systems. (A) Passive diffusion. (B) Facilitated diffusion. (C) Primary active transport (left) and secondary active transport (right). Please click here to view a larger version of this figure.
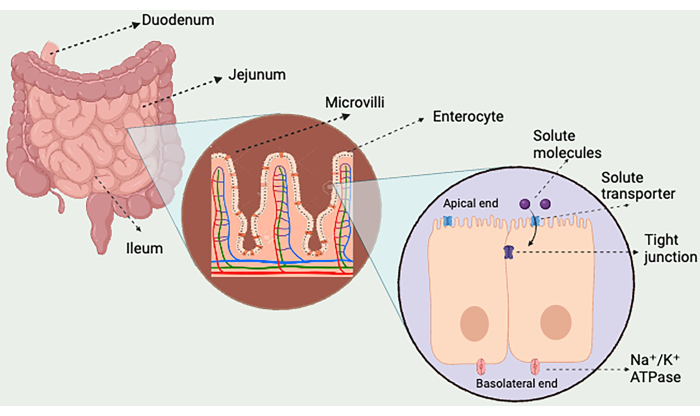
Figure 2: Organization of enterocytes in intestinal villus. This figure focuses on the small intestine and illustrates the three distinct parts, i.e., Duodenum, Jejunum, and Ileum. The small intestine further has some finger-like projections called the villi, which increase the surface area for absorption of ingested food. Microvilli have cells called enterocytes that contain the specific transporters for amino acid absorption. Tight junctions prevent the movement of solutes in between the cells. Please click here to view a larger version of this figure.
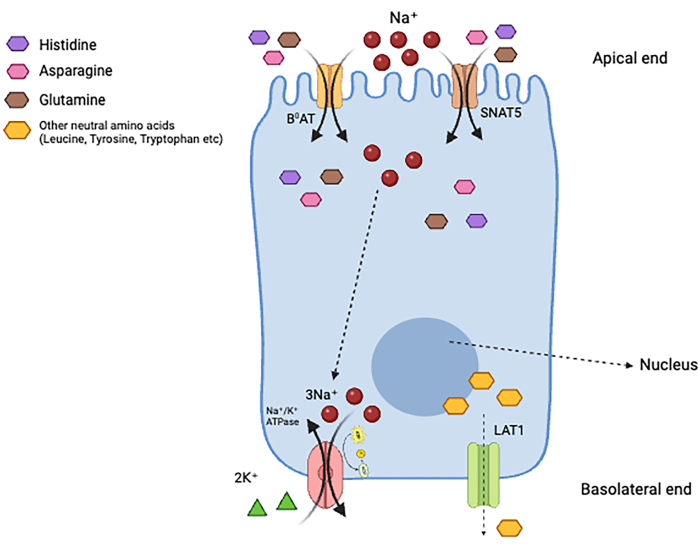
Figure 3: An enterocyte with amino acid transporters. The cartoon represents one enterocyte showing the apical and basolateral ends. The apical end has transporters such as the B0AT and the SNAT5 proteins, and the basolateral membrane contains the LAT1 transporter. Together, these proteins are responsible for the uptake of histidine from the intestinal lumen to the portal circulation. Please click here to view a larger version of this figure.
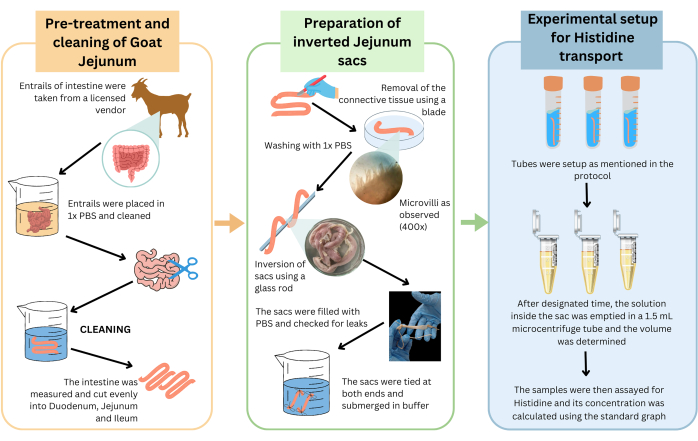
Figure 4: Schematic representation of the protocol demonstrating membrane transport of histidine using goat intestine inverted sacs. Goat intestines were procured, pre-treated, and cleaned as described. Briefly, the jejunum was excised, and inverted sacs were prepared. These sacs were used to set up the experiment to quantify histidine uptake in various salt concentrations. The villi were viewed at 400x magnification using a light microscope. Detailed procedure is provided in the text. Please click here to view a larger version of this figure.
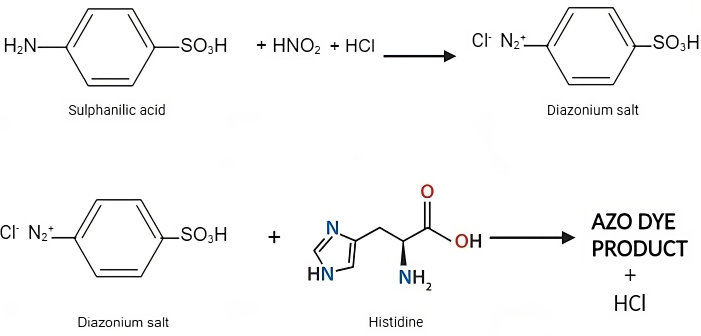
Figure 5: Reaction for Pauly's method. Pauly's test involves the formation of diazonium ions in a reagent containing sulphanilic acid in concentrated hydrochloric acid. Under alkaline conditions, histidine reacts with the diazonium salt, resulting in the formation of a red-colored compound whose absorbance can be measured at 490 nm using a spectrophotometer. Please click here to view a larger version of this figure.
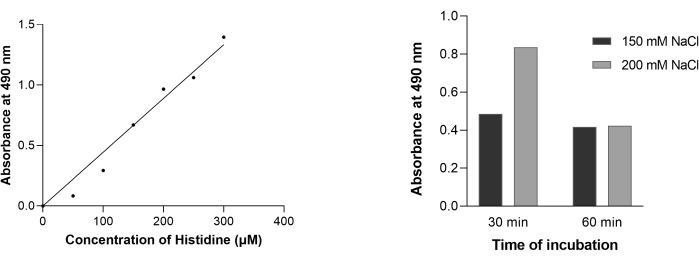
Figure 6: Estimation of histidine by Pauly's method. (A) Standard curve for histidine. Varying concentrations of histidine ranging from 0-300 μM were subjected to Pauly's reaction, and the complex formed was measured at wavelength 490 nm. (B) Transport for histidine in intestinal sacs was followed for 30 min and 60 min in the presence of 150 mM (solid black) and 200 mM NaCl (solid grey) salt solution. The concentration of histidine inside the inverted sacs was estimated using the standard curve (Figure 6A). The values are reported with respect to the control (0 mM NaCl). Three technical replicates were performed, and representative data are presented. Please click here to view a larger version of this figure.
Table 1: Experimental setup for intestinal mobility of Histidine. This table shows the incubation of intestinal sacs in different concentrations of sodium chloride over two time points. Please click here to download this Table.
Table 2: Preparation of standard curve for Histidine. This table shows the different steps and components for Pauly's reaction with known concentrations of histidine ranging from 0-300 μM. Please click here to download this Table.
Discussion
Membrane transport is one of the most fundamental concepts taught to undergraduate students of all major biological science disciplines, basic or applied. Traditionally, movement across membranes has been visualized using metabolites labeled with radioactive isotopes. However, these methods are extremely hazardous and not feasible for teaching or learning. While experiential learning is the best pedagogical technique to understand such complex concepts, it is a challenge that is further compounded by the lack of infrastructure, expensive fluorescence-based dyes/metabolites and most importantly, the ban on animal dissection experiments. Thus, there is a need to develop tools and alternative methods that are not only safe but also inexpensive and feasible in an undergraduate laboratory setting. Here, a novel method for teaching membrane transport was developed for undergraduate students. The reported protocol, an adaptation from Agar et al. 19548, demonstrates unidirectional active membrane transport of histidine across goat intestine cells. This study was undertaken to demonstrate the use of a colorimetry-based assay to visualize histidine absorption across intestinal membranes. It is simple, reproducible, and comprehensible for students and can be done in any laboratory setting.
The inverted intestinal sac model was first developed by Wilson and Wiseman10, which was later improved to increase the viability of the tissues. The mechanisms and kinetics of drug absorption have been elucidated using gut inverted sacs11. The advantages of this model include a large area for absorption; however, tissue viability is one of the limiting parameters. It has been reported that the intestinal tissue remains viable and metabolically active under physiological conditions for ~2 h11. Another potential caveat of this approach that needs to be taken into consideration is the inability to remove the muscularis mucosa and serosa from the inverted sac preparations. The binding of certain compounds to the muscularis layer may limit or underestimate the transport of these compounds. Though the inverted intestinal sac model is both sensitive and specific, a variety of factors could impact the results. Some such factors include age, sex, species, diet, and disease state of the animal, as well as the segment of the intestinal used for analysis (Ileum, Jejunum, Duodenum, and Colon). Environmental factors such as pH, aeration, and temperature would also impact the results12.
It has been shown that delay in the harvesting of the intestine and animal state (live/dead) affects active transport in the duodenal segments of the rat intestine. Harvesting from anesthetized animals ensures minimal time delay, avoiding transporter deterioration13. However, in light of the ban on animal dissections in universities, intestines from freshly sacrificed animals were used in our experimental setup. While this protocol can be successfully used to establish a qualitative uptake of a metabolite across the enterocytes, its use in studies involving membrane transport kinetics is limiting. The most critical step of this protocol is the successful preparation of leak-proof inverted intestinal sacs with viable enterocytes. The delicate nature and the viability of the villus and the enterocytes pose a challenge, one that can be overcome with practice. Furthermore, given that the starting material is not from any inbred, syngenic animal population, variability in results between experiments is a significant drawback. This may be overcome with the use of multiple replicates from the same animal. Keeping in mind these considerations, the method presented in this study is strictly to be used as a pedagogical tool in undergraduate practical training.
The protocol outlined was successfully used to demonstrate the transport of histidine. The unidirectional uptake of both sodium and histidine into the inverted sacs indicates a symport type of secondary active transport. Intestinal absorption of neutral amino acids follows vectorial kinetics that is dependent on the concentration gradient set up by the Na+ K+ ATPase. A similar setup maintains an intracellular negative membrane potential and an extracellular sodium concentration of 150 mM. Both these drive the active transport of neutral amino acids by B0AT at the apical end and subsequent movement out of the enterocyte at the basolateral end by LAT1. Interestingly, at higher salt concentrations (200 mM), there was no significant difference in the absorption as a function of time. At this point, we cannot comment on the reason for such an observation; however, the same experimental setup may be expanded to include multiple sodium concentrations with variable time points and controls with 150 mM choline chloride to display sodium-dependent transport and possible contribution due to saturation.
In principle, inverted jejunum sacs from goat/pig/rat/mouse can be used to demonstrate intestinal absorption of a variety of dietary metabolites, provided there is a biochemical assay for estimation. Additionally, the effect of other components that could potentially interfere with intestinal absorption or membrane transport inhibitors can also be analyzed. Inverted sacs have been efficiently used as a tool in pharmaceutical research to study in vitro drug absorption, intestinal metabolism of drugs, and the role of the transporter in drug absorption. Finally, this technique is an example of an ex vivo demonstration of membrane transport and does not rely on cell-free extract (in vitro) but on a tissue sample. However, this advantage is offset by the availability of uninfected goat/pig/rat intestines, which can be a limiting factor.
Amongst the many pedagogical tools available to a teacher to enhance the learning process, experiential learning or 'learning by doing' is a philosophy and methodology of teaching that is the most progressive and effective method of instruction that provides students an opportunity to get a holistic understanding of lecture topics. Experiential learning ensures the hands-on engagement of the students as well as the instructor, who provides support and mentorship during this active learning process14. It serves as an excellent alternative to traditional theoretical classroom instruction, particularly in certain heavy and integrative concepts like membrane transport that students often find difficult to envision. Such exercises would also encourage interactive and collaborative learning from both peers as well as mentors. The present initiative is a step forward in the direction of creating and enabling a learning experience that is not only informative but also memorable and can be transformative to a student, significantly impacting the overall learning experience.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study has been supported by the Department of Biochemistry, Sri Venkateswara College, University of Delhi. The authors thank the laboratory staff for their support.
Materials
| 1.5 mL Microcentrifuge Tubes | TARSONS | 500020 | |
| 10 mL Test Tubes | BOROSIL | 9800U04 | |
| 50 mL Sterile Falcon Tubes | TARSONS | 546041 | |
| 500 mL Beaker | BOROSIL | 10044977 | |
| 500 mL Conical Flask | BOROSIL | 691467 | |
| D-Glucose | SRL | 42738 | |
| Digital Spectrophotometer | SYSTRONICS | 2710 | |
| Ethanol | EMSURE | 1009831000 | |
| Finpipettes | THERMOFISHER | 4642090 | |
| Glass Stirrer Rod | BOROSIL | 9850107 | |
| L-Histidine | SRL | 17849 | |
| NaCl | SRL | 41721 | |
| Nitrile Gloves | KIMTECH | 112-4847 | |
| Petri Dish | TARSONS | 460090 | |
| Phosphate Buffered Saline (ph 7.4) | SRL | 95131 | |
| Pipette Tips | ABDOS | P10102 | |
| Sodium Carbonate | SRL | 89382 | |
| Sodium Nitrate | SRL | 44618 | |
| Sodium Phosphate Dibasic (anhydrous) | SRL | 53046 | |
| Sodium Phosphate Monobasic (anhydrous) | SRL | 22249 | |
| Sulphanilic Acid | SRL | 15354 |
Riferimenti
- Nelson, D. L., Cox, M. M. . Lehninger Principles of Biochemistry. , (2017).
- Stillwell, W. Membrane Transport. An Introduction to Biological Membranes. , (2013).
- Fick, A. V. On liquid diffusion. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 10 (63), 30-39 (1855).
- Sherwood, L. . Introduction to Human Physiology. , (2013).
- Bröer, S. Intestinal amino acid transport and metabolic health. Annu Rev Nutr. 43, 73-99 (2023).
- Avissar, N. E., Ryan, C. K., Ganapathy, V., Sax, H. C. Na+-dependent neutral amino acid transporter ATB0 is a rabbit epithelial cell brush-border protein. Am J Physiol Cell Physiol. 281 (3), C963-C971 (2001).
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 88 (1), 249-286 (2008).
- Agar, W. T., Hird, F. J. R., Sidhu, G. S. The uptake of amino acids by the intestine. BBA – Biochim Biophys Acta. 14 (1), 80-84 (1954).
- Pauly, H. Über die Konstitution des Histidins: I. Mitteilung. Biological Chemistry. 42 (5-6), 508-518 (1904).
- Wilson, T. H., Wiseman, G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 123 (1), 116-125 (1954).
- Barthe, L., Woodley, J. F., Kenworthy, S., Houin, G. An improved everted gut sac as a simple and accurate technique to measure paracellular transport across the small intestine. Eur J Drug Metab Pharmacokinet. 23 (2), 313-323 (1998).
- Alam, M. A., Al-Jenoobi, F. I., Al-Mohizea, A. M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J Pharm Pharmacol. 64 (3), 326-336 (2012).
- Pento, J. T., Mousissian, G. K. Time-dependent deterioration of active transport in duodenal segments of rat intestine. J Pharmacol Methods. 20 (1), 9-14 (1988).
- Williams, L., Sembiante, S. F. Experiential learning in U.S. undergraduate teacher preparation programs: A review of the literature. Teach Teach Educ. 112, 103630 (2022).

