Streamlined Sampling and Cultivation of the Pelagic Cosmopolitan Larvacean, Oikopleura dioica
Summary
Oikopleura dioica is a tunicate model organism in various fields of biology. We describe sampling methods, species identification, culturing setup, and culturing protocols for the animals and algal feed. We highlight key factors that helped strengthen the culture system and discuss the possible problems and resolutions.
Abstract
Oikopleura dioica is a planktonic chordate with exceptional filter-feeding ability, rapid generation time, conserved early development, and a compact genome. For these reasons, it is considered a useful model organism for marine ecological studies, evolutionary developmental biology, and genomics. As research often requires a steady supply of animal resources, it is useful to establish a reliable, low-maintenance culture system. Here we describe a step-by-step method for establishing an O. dioica culture. We describe how to select potential sampling sites, collection methods, target animal identification, and the set-up of the culturing system. We provide troubleshooting advice based on our own experiences. We also highlight critical factors that help sustain a robust culture system. Although the culture protocol provided here is optimized for O. dioica, we hope our sampling technique and culture setup will inspire new ideas for maintaining other fragile pelagic invertebrates.
Introduction
Model organisms have been instrumental in addressing many biological questions including those relating to development, genetics, and physiology. Moreover, additional model organisms facilitate new discoveries and therefore are crucial to achieve a greater understanding of nature1,2. Marine zooplankton are diverse groups of organisms that play an important role in ocean ecosystems3,4,5,6. Despite their abundance and ecological importance, gelatinous organisms such as planktonic tunicates are often under-represented in plankton biodiversity studies because their transparency and fragility make field collection and identification challenging7,8. Adapted sampling techniques and laboratory culturing allow closer observation of the animals in vitro, which has furthered the knowledge in the biology of planktonic tunicates9,10,11,12.
Larvaceans (Appendicularians) are a class of free-swimming marine tunicates comprising around 70 described species worldwide8,13. As they are one of the most abundant groups within zooplankton communities14,15,16,17, larvaceans represent a primary food source for larger planktonic organisms such as fish larvae18,19. Unlike ascidians-the sessile tunicates-larvaceans retain a tadpole-like morphology and remain planktonic throughout their lives20. Each animal lives inside a self-built, intricate filter-feeding structure known as a house. They accumulate particulates in their houses by creating water currents through the undulating motion of their tails21. Clogged houses are discarded throughout the day, some of which form carbon aggregates and eventually sink to the seabed22; thus, larvaceans play a major role in global carbon flux23. Most species are reported to live in the pelagic zone within the upper 100 m of the water column13; however, the giant larvacean Bathochordaeus is known to inhabit the depths of 300 m24. A study on Bathochordaeus in Monterey Bay, California revealed that the animals also serve as a biological vector of microplastics, suggesting a potential importance in understanding the role of appendicularians in the vertical transport and distribution of microplastics in the oceans25.
Oikopleura dioica, a species of larvacean, has attracted attention in recent years as a model organism due to several remarkable characteristics. It is commonly reported throughout the world’s oceans. It is especially abundant in coastal waters26, which allows easy sampling from the shore. Long-term, stable culturing is possible with both natural and artificial seawater27,28,29. Temperature dependent generation times are as short as 4-9 days in laboratory conditions. It has high fecundity with each female capable of producing >300 eggs throughout the year. As a tunicate, it occupies an important phylogenetic position for understanding chordate evolution30,31. At 70 Mb, O. dioica has the smallest identified genome amongst all chordates32. Among larvaceans, O. dioica is the only described non-hermaphroditic species thus far33.
The first successful O. dioica culture with laboratory grown microalgae was reported by Paffenhöfer34. The original culture protocol using synchronous motors and paddles was developed by Fenaux and Gorsky35 and later adopted by multiple laboratories. More recently, Fujii et al.36 reported O. dioica culturing in artificial seawater, a robust culture system and field collection were described by Bouquet et al.27 and an optimized protocol for a simplified, affordable system was reported by Marti-Solans et al.29. Aside from the traditional Oikopleura culture system, a newly reported design with a double tube rearing tank also has the potential to culture Oikopleura sp.37.
We present a detailed protocol for initiating an O. dioica monoculture based on a combination of protocols developed by major Oikopleura research groups at the Sars International Centre for Marine Molecular Biology27, the University of Barcelona29, Osaka University28, and our own observations. In previously published culture protocols, detailed information regarding the composition of algal media, shore sampling techniques, and Oikopleura identification were only roughly described, leaving a lot of ambiguity. Here, with the aid of visual information in the video protocol, we have assembled all the critical information needed to set up an O. dioica culture from the ground up in a straightforward, step-by-step manner. We describe how to distinguish O. dioica from another commonly reported species, O. longicauda, which is one of the most challenging steps. Although the existing culture systems are applicable for the cultivation of O. dioica worldwide, we highlight the importance of protocol adjustment based on local environmental conditions. The presented information combines widely published data as well as knowledge gained through experience. The current protocol is ideally suited for researchers interested in establishing a culture from scratch.
Protocol
1. O. dioica culture facility
- Water filter system (Figure 1)
- Collect natural seawater from a harbor at 2-3 m depth. Pass the seawater through a sand filter (pore size 1.4 mm) and transport to a shared reservoir tank in the laboratory. Use a canister filter to circulate the water in order to maintain the water quality in the shared reservoir tank.
- In a culture room, set up a multi-step filter system consisting of a 100 L reservoir tank with a magnetic drive pump, 5 µm and 1 µm polypropylene wound cartridge filters, and a UV sterilizer (100 V) (Figure 1).
- Transfer the seawater from the shared reservoir tank to the culture room reservoir tank. Pass the seawater through a 25 µm filter unit (Figure 1A,B) before entering the culture room reservoir tank. Circulate the seawater through 5 and 1 µm filters overnight to thoroughly remove particles that could potentially hinder the development of animals.
NOTE: An additional filter with larger mesh size (25-50 µm) is useful to prevent larger particles from clogging the cartridge filters with smaller mesh sizes. The filtered seawater (fSW) is ready for use the following morning.
- Oikopleura culturing unit (Figure 2)
- Maintain animals in 5 or 10 L round, transparent plastic beakers.
- Place culture beakers on a steady, two-level stainless-steel shelving unit (L x W x H = 150 cm x 45 cm x 90 cm) with a 5 mm thick, transparent acrylic surface board.
- Position white fluorescent lights underneath the acrylic surface to illuminate the animals from the bottom of the beakers.
- Place a black plastic sheet behind the beakers. The black sheet creates contrast and enhances visualization of the transparent animals.
- Connect synchronous electric motors to acrylic paddles (L x H = 8 cm x 27 cm) (Supplemental File 2). Suspend the paddles in the culture beakers from parallel rails running along the length of the shelving unit (Figure 2A).
- Switch on the motors to generate a gentle circular motion in the beakers at 15 RPM.
NOTE: Animals in their cellulose houses are neutrally buoyant; however, water circulation helps to keep eggs, larvae, and algal food to be suspended and evenly distributed in the culture beakers.
- Automatic dosing pump (Optional)
NOTE: An automatic feeding unit reduces staffing requirements, especially during the weekends.- Calibrate the volume of dispensing liquid from an automatic dosing pump according to the manufacturer’s instructions.
- Use 50 mL tubes as algal reservoirs.
- Drill two 5 mm holes on the caps of 50 mL tubes to pass through airline tubing. Connect one tube to a standard aquarium air pump to introduce air bubbles, and the other tube to the inlet port of dosing pump (Figure 2B).
NOTE: Introducing a thin stream of air bubbles helps to prevent algae from settling on the bottom of the tubes. - Program the time and volume of algal feed to be dispensed on a given day.
- Algae station
- Use a shelving unit (L x W x H = 90 cm x 46 cm x 115 cm) to place four 1 L round bottom flasks containing algal working cultures (See step 2.1).
- Back-illuminate the working cultures by placing fluorescent lights behind the flasks.
- Seal flasks with two-hole rubber stoppers.
- Pass a 1 mL disposable pipette through the rubber stopper. Use airline tubing to connect the pipette to an aquarium air pump. Introduce a stream of air bubbles in the flask.
2. Microalgal food
- Initiating algal cultures
NOTE: Maintain three sets of cultures (stock, sub-, and working cultures) for three microalgal species, Chaetoceros calcitrans, Isochrysis sp., Rhinomonas reticulata, and one species of cyanobacteria, Synechococcus sp.. Stock and sub-cultures are used as back-ups. The working culture is used for daily feeding.- Prepare reagents necessary for the cultivation of microalgae and cyanobacteria (Table 1).
- To initiate stock culture, autoclave (121 °C, 25 min) 60 – 80 mL of fSW in a 100 mL Erlenmeyer flask. Aseptically inoculate specified amount of modified Conway medium27 and microalgae (Table 2). For example, to inoculate a stock culture of C. calcitrans, autoclave 60 mL of seawater, aseptically inoculate 30 µL each of the vitamin and solution A, 15 µL of sodium silicate, 60 µL of streptomycin, and 30 µL of C. calcitran from the previous stock culture.
NOTE: R. reticulata turns from reddish-pink to orangish-brown when exposed to too much light. Move them away from light once they have started to turn from clear to light pink. - Maintain the stock culture in an incubator set at 17 °C with continuous lighting. After about 10 days, the culture changes color to indicate algal growth (Figure 3). Once the colors appear, move them to 4 °C for long-term storage for up to 1 month.
- On a clean bench, aseptically inoculate a sub-culture from the stock culture (Table 2). Incubate at 17 °C with continuous lighting. After algal colors appear, continue to store them in the incubator up to 2 weeks.
- Inoculate working culture from sub-culture (Table 2). Seal the flask with a rubber cap and insert 1 mL disposable pipette. Move the flask to the algae station and maintain at room temperature with a 8 h photoperiod. Supply with constant aeration. Renew the working culture every 4 days.
- Stir stock and sub-cultures twice a day by swirling.
NOTE: Long-term storage of algal culture on solid media and cryopreservation are possible up to 3 months and 1 year, respectively29.
- Creating algal growth curves (Optional)
NOTE: Accurate assessment of feeding quantity is important to maintain a stable culture of O. dioica. We created growth curves for two primary algal food species, Chaetoceros calcitrans and Isochrysis sp.- Prepare C. calcitrans and Isochrysis sp. working cultures (Table 2).
- For each species of working culture, sample three separate times and measure absorbances at 660 nm using a spectrophotometer. Take the average measurements of the triplicates from each working culture.
- Following the manufacturer's instructions for an automated cell counter, prepare algal samples for counting. Count each sample three times. Take the average of three counts to determine the total number of cells present in each sample.
- Continue to count daily until approximately 50 average measurements are recorded.
- Create growth curves for both algal species (Figure 4).
3. Field collection of wild Oikopleura spp.
- Modified plankton net (Figure 5)
NOTE: The key to successful sampling of Oikopleura spp. is the slow towing of a plankton net with a weighted, non-filtering cod-end. Figure 5 shows a schematic diagram of a modified plankton net.- Replace the cod-end of a hand-held plankton net with a modified 500 mL screw-top wash bottle.
- Drill a 3 cm diameter hole in the 4 cm diameter screw-top of the wash bottle to allow water and animals to enter the cod-end.
- Fit the bottle cap at the end of plankton net. Wrap it tightly with electric tape. Secure the cap further with a stainless-steel hose clamp.
- Attach a 70 g weight to the outside of the modified cod-end with zip ties.
- Attach safety leash to further secure the cod-end.
- Selecting collection sites (Figure 6)
NOTE: All sample collections were approved by the OIST Fieldwork Safety Committee. There might be seasonal variation in the presence of Oikopleura spp. depending on the location (Figure 6). Avoid sampling immediately after extreme weather events such as severe rainstorms.- Use the satellite view on a map website to identify potential sampling sites. We focused on harbors and fishing piers that are easily accessible by car and located inside bays or near ocean drop-offs where plankton tend to accumulate: Ishikawa harbor in Kin Bay, Okinawa, Japan (GPS: 26°25'39.3"N 127°49'56.6"E).
- Visit potential sampling locations to assess shore accessibility and safety of each site. Obtain collection permit from local authorities as needed.
- Sampling procedure
- Cast the plankton net into the sea and allow the cod-end to sink 1-2 m below the water’s surface.
- Tow the net horizontally by hand at 50-100 cm s-1. Continue towing by walking back and forth for 2-5 minutes. Adjust towing time according to the abundance of phytoplankton in the harbor, with shorter tow when there are more phytoplankton.
NOTE: Larvaceans are fragile animals. Fast towing or repeated casting of the net could damage animals trapped in the cod-end. - Gently lift the net. Slowly transfer the contents of the cod-end into a 500 mL round glass bottle. Completely fill the sample bottle with seawater to avoid air bubbles.
NOTE: The presence of Oikopleura spp. can be confirmed by viewing sample bottles against a black background. Most animals abandon their houses while being collected. Therefore, microscopic observation is needed for species-level identification. - Repeat sampling until three 500 mL bottles are collected.
- Measure salinity, temperature, and chlorophyll a using a CTD profiler to record the range of physical parameters where animals naturally exist.
- Collect 10-15 L of surface seawater in a bucket to acclimatise animals in the laboratory setting.
4. Animal isolation and identification (Figure 7, Figure 8)
- Oikopleura spp. identification
NOTE: Other planktonic organisms that may resemble Oikopleura spp. at first glance include chaetognaths, Fritillaria spp., nematodes, fish larvae with yolk-sacs, and Ciona spp. larvae.- To acclimatize animals to laboratory conditions, transfer each 500 mL sample to a 10 L beaker containing 1:1 ratio of surface seawater from the sampling location and filtered seawater (fSW) maintained in the lab (Figure 7A,B). Adjust the volume of the beaker to 5-10 L depending on the concentration of plankton sample.
NOTE: If the plankton sample contains unwanted debris, run through a coarse filter (mesh size ~600 µm) before transferring to a 10 L beaker. - Use a paddle attached to a synchronous electric motor (15 RPM) and keep the plankton in suspension overnight (step 1.2.5).
- Identify Oikopleura spp. by looking for 1-2 mm long, tadpole-shaped animals undulating their tails inside a spherical, translucent house. Some animals may be temporarily free-swimming without the houses. Gently transfer ~5 animals to an empty Petri dish using a blunt-end pipette.
- For genus identification, evict animals from their houses by gently poking the house with a transfer pipette.
- Observe houseless animals under a 20-40x dark-field microscope and confirm Oikopleura spp (Figure 8).
- To acclimatize animals to laboratory conditions, transfer each 500 mL sample to a 10 L beaker containing 1:1 ratio of surface seawater from the sampling location and filtered seawater (fSW) maintained in the lab (Figure 7A,B). Adjust the volume of the beaker to 5-10 L depending on the concentration of plankton sample.
- O. dioica identification
NOTE: O. dioica can be visually identified by the presence of fully mature males and females or two large subchordal cells located on the distal half of their tails. The distance between two subchordal cells may vary between individuals.- Next, check if there is a fully matured Oikopleura with a gonad filled with eggs (Figure 8A) or sperm (Figure 8B). If the animal only possesses eggs or sperm, skip to step 4.2.3 as it is O. dioica, the only described non-hermaphroditic species.
- If the animal is immature (Figure 8C), look for two subchordal cells at the end of its tail (Figure 8D).
- Once the species is confirmed, transfer it to a new Petri dish. Repeat steps 4.1.3-4.2.2 until 10-20 individuals are confirmed at species-level.
NOTE: For easier identification, anesthetize animals in a Petri dish containing 0.015% tricaine methanesulfonate (MS222) in fSW. - If no O. dioica are found, keep the beakers suspended for an extra day or two. There might be immature O. dioica that will continue growing and become easier to be detected. If none appear after a week, discard the sample, and try sampling again.
5. Cultivation protocol for O. dioica
- Initiating an O. dioica monoculture from a field collected sample (Figure 7)
NOTE: Algal food is prepared daily from working cultures and each monoculture beaker is fed three times a day at 9 AM, 12 PM, and 5 PM, respectively (See step 5.2). The animals are maintained at 23 °C. Under these conditions, the Okinawa O. dioica lifecycle is 4 days (Figure 7C).- To initiate a monoculture of O. dioica, isolate 120 animals and transfer to a new beaker containing 5 L of fresh fSW (Figure 7B,C).
- The following morning, look for fully mature males with yellow gonads and females with eggs that appear as golden spheres (Figure 8A,B).
- Make a spawning beaker by gently transferring 15 males and 30 females to a new beaker containing 2.5 L of fresh fSW with a 5 mL blunt-end pipette.
NOTE: If there are not enough males and females, transfer as many adults as possible to a beaker containing 1 L of fSW and let them spawn naturally. To minimize physical stress to animals during manual transfer, they should be slowly siphoned and released under the water surface. - Let the animals spawn naturally to initiate the next generation. Tailed larvae should appear approximately 3 hours after fertilization.
NOTE: Spawning is performed by fully matured animals abandoning their houses, swimming towards the surface water, and releasing their gametes. Successful fertilization can be confirmed by extracting 5-10 mL of seawater from the bottom of the spawning beaker and identifying eggs with cleavages under a microscope. - On the first morning post-spawning (Day 1), a new generation of animals with inflated houses should appear in the beaker. Use a 500 mL hand-held beaker to gently transfer the contents of the spawning beaker into a new beaker containing 7.5 L of fresh fSW (making a total of 10 L). Pour at an angle to avoid a splashing motion.
- On the second morning (Day 2), manually transfer 150 animals to a new beaker containing 5 L of fresh fSW.
- On the third morning (Day 3), manually transfer 120 animals to a new beaker with 5 L of fresh fSW.
NOTE: In order to synchronize the development of animals, it is important to select individuals with similar sizes during the manual transfer on day 2 and 3. A maximum of 10 animals can be siphoned in a single transfer. - On the fourth morning (Day 4), fully matured animals should appear. Repeat step 5.1.3 to close the lifecycle.
NOTE: An automated feeding pump can be set to feed the animals at 5 PM on weekends without the presence of culturing staff.
- Daily preparation of algal food from working culture
- Measure the absorbance of the working culture at 660 nm.
- Based on the Daily Feeding Chart, find out how many algal cells need to be fed for the animals of specific size (Table 3).
- Using the algal growth curves (Figure 4), solve the equations below to calculate the volume of algal food (mL) required on a given day.
- To calculate the volume of a particular algae needed for a specific day and feeding time, use the following equation:
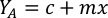
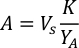
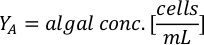
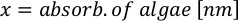
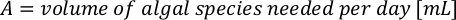

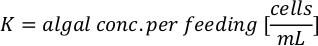
Where YA is the algal concentration on a given day and A is the volume of algae needed per feeding. Furthermore, the linear relationship between YA a x, the values for intercept (c) and slope (m) are shown in Figure 4. Refer to Table 3 for K values. - For example, to calculate the volume of Isochrysis sp. needed at a 9 AM feeding of Day 3 animals maintained in a 5 L culture and with the algal absorbance of 0.234 (measured at 660 nm), the following was computed:
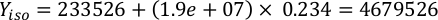
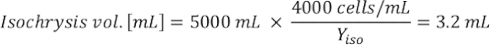
NOTE: Store these equations in a spreadsheet so the daily feeding amount is automatically calculated based on absorbance measurements, the size of the animals, and the volume of culture seawater (Supplemental File 1).
- To calculate the volume of a particular algae needed for a specific day and feeding time, use the following equation:
- Transfer the calculated volume of algae to 50 mL tubes, centrifuge at 5000 x g for 5 min at 20 °C.
- Remove the supernatant. Fill the tubes back up to the original volume with fresh fSW, replacing old algal media.
- Store prepared food in fridge until ready to be used for the next feed. Discard the old algal food after new food is prepared the next morning.
- Activated charcoal (Optional)
NOTE: 10 g of activated charcoal is added to each culture beaker to maintain water quality. The charcoal can be reused up to four times. Open the charcoal bag slowly to avoid charcoal dust from entering the culture beakers.- Transfer ~700 g of activated charcoal in a container. Soak in fresh water (FW) for 48 hours and allow them to settle on the bottom.
- Rinse with FW to remove residual charcoal dust.
- Boil charcoal in FW for 15-20 min. Remove from heat and allow to cool.
- Rinse until most charcoal dust is removed, and the water becomes clear.
- Store clean charcoal in 2 L beaker containing fSW. Cover the beaker to prevent dust from entering.
- Add charcoal to each new beaker before transferring the animals.
Representative Results
Oikopleura can be collected from a boat or from a harbor by slow, gentle towing of a 100 µm mesh plankton net with a non-filtering cod-end (Figure 5). Due to the fragile nature of the animals, it is important to avoid any movement that could cause physical stress, such as rough handling of the net or splashing due to a trapped air pocket in the sample jar.
It is important to understand the seasonal pattern of local Oikopleura populations as well as the accompanying fluctuations in the physical characteristics of the water at a sampling site. Sampling between 2015 and 2019 revealed consistent seasonal variation in the presence of O. dioica in Ishikawa and Kin harbors in Okinawa (Figure 6). Surface seawater temperature appears to be a major factor. O. dioica was the dominant species when surface seawater reached ≥28 °C, and O. longicauda coexisted with O. dioica at temperatures between 24 °C and 27 °C; however, O. longicauda dominated below 23 °C (Figure 6A). Gradual change in salinity after several consecutive days of heavy rain did not correlate with the abundance of O. dioica (Figure 6B).
Using the sampling procedures described above, most O. dioica we recovered were between day 2 and 3 of their 4-day lifecycle (Figure 7C). Mature males were recognized by the yellow coloration of gonads whereas female gonads shimmered gold from eggs that were 70-80 µm in diameter (Figure 8A,B). Immature O. dioica were confirmed by two subchordal cells on their tails (Figure 8D). Another dominant species in the local waters, O. longicauda, were similar in size and morphology. We used the following criteria to distinguish O. longicauda from O. dioica38,39,40: a lack of subchordal cells in the tail, the presence of velum in the trunk, and the presence of a hermaphrodite gonad (Figure 8E,F). The differing tail morphologies are also useful for distinguishing O. longicauda from O. dioica. When an intact naked animal without the house was oriented laterally, the tail of O. longicauda was more straight with less curvature, giving it a “stiffer” appearance compared to that of O. dioica.
The three most important factors for establishing a stable Oikopleura culture system are (i) maintaining high water quality, (ii) identifying the optimal feeding regime, and (iii) setting up a spawning beaker with sufficient numbers of males and females. The introduction of a multi-step filter system (Figure 1) improved the water quality and stability of the culture. A filtration system is not necessary for artificial seawater; however, the cost, availability, and convenience of natural seawater makes it a better option for labs situated near the coast. To establish the feeding regime, we recommend measuring algal growth curves that apply to individual laboratory settings, since temperature and light conditions vary greatly. We combined the growth curves with previously published feeding schedules to optimize algal feed concentrations and compositions27 (Figure 4). We also follow a strict algal inoculation schedule to maintain a fresh supply of algal food (Table 2). The automated feeding system allows us to maintain a consistent daily feeding schedule without the presence of culturing staff (Figure 2B).
Once optimal seawater and feeding conditions are achieved, it is important to initiate new generations by creating a spawning beaker with 15 males and 30 females in 2.5 L of fSW. This ensures a good concentration of Day 1 animals the following morning, which is sufficient to isolate 150 animals on Day 2, 120 on Day 3 and 45 mature adults on Day 4 for spawning. If there are not enough males and females on Day 4, collect and transfer as many mature individuals as possible to 1 L of fSW and let them spawn naturally in the hope that there will be enough larvae to carry onto the next generation. Following the provided protocol, the lifecycle of O. dioica is 4 days at 23 °C (Figure 7C). We have reliably established six independent wild populations of O. dioica, all of which lasted more than 20 generations.
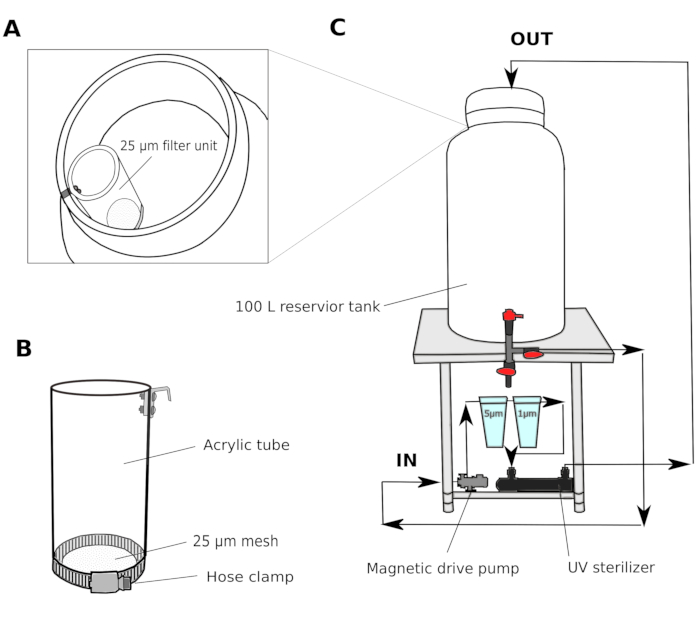
Figure 1: Schematic of seawater filter system.
(A and B) Seawater is initially filtered through a 25 µm filter unit before entering the reservoir tank (C) A magnetic drive pump is used to draw seawater from the reservoir tank. The seawater is then pushed through two polypropylene filters and a UV sterilizer before returning to the reservoir tank. Please click here to view a larger version of this figure.
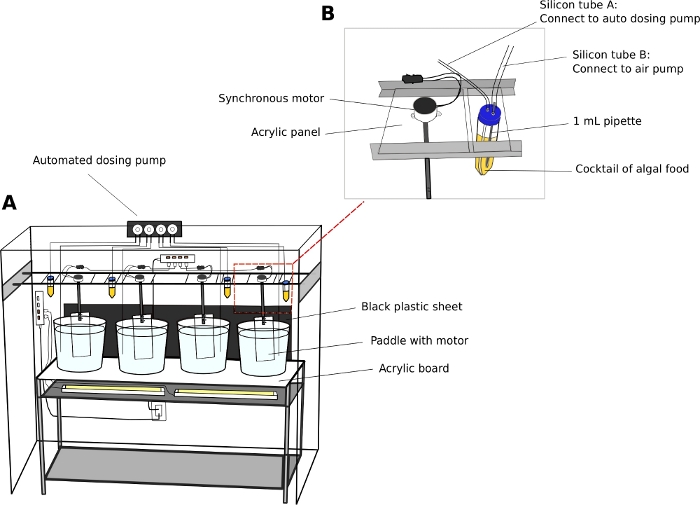
Figure 2: Culture system for O. dioica.
(A) Overview of the culture system (B) Close-up view of synchronous motor and algae reservoir for the automated dosing pump. Inner diameters of silicon tube A and B are 2 mm and 4 mm, respectively. Please click here to view a larger version of this figure.

Figure 3: Stock cultures for O. dioica.
From left- C. calcitrans, Isochrysis sp., Synechococcus sp., and R. reticulata after being grown at 17 °C under continuous light for ~10 days. Please click here to view a larger version of this figure.

Figure 4: Algal growth curve for two of the major food species, C. calcitrans and Isochrysis sp..
Scatter plots of optical density (OD) at 660 nm and total cell concentrations for (A) C. calcitrans and (B) Isochrysis sp.. Each point represents the average of three measurements. A cell counter was used to determine the percentage of viable cells and total cell concentrations (cells/mL). Measurements were recorded for 20 days (n = 47). Please click here to view a larger version of this figure.
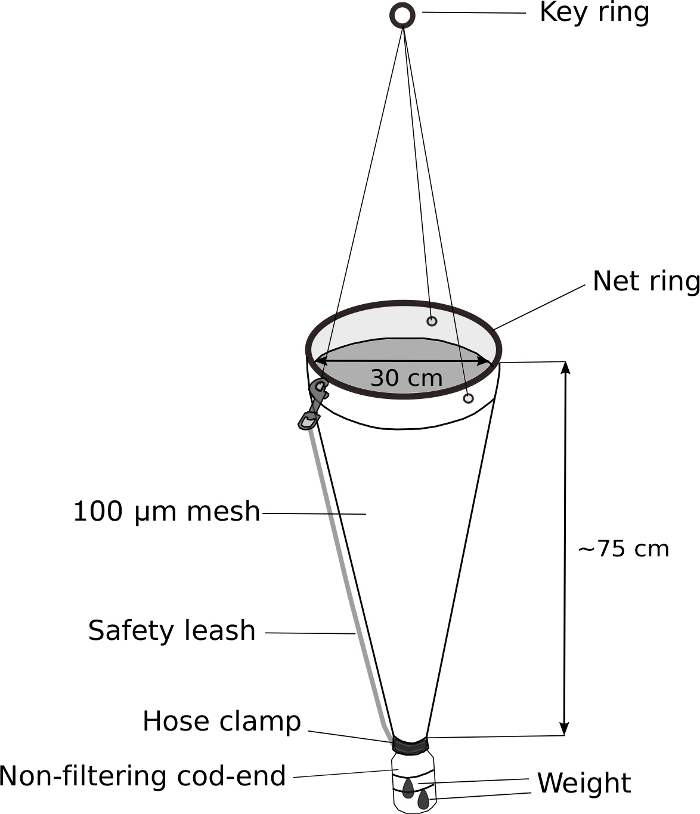
Figure 5: Modified plankton net for Oikopleura sampling.
The cod-end of a hand-held plankton net (100 µm mesh) is replaced with a 500 mL wash-bottle. A 70 g weight is attached to the cod-end. Approximately 5 m of rope is attached to the key ring. A safety leash is attached to further secure the cod-end. Please click here to view a larger version of this figure.
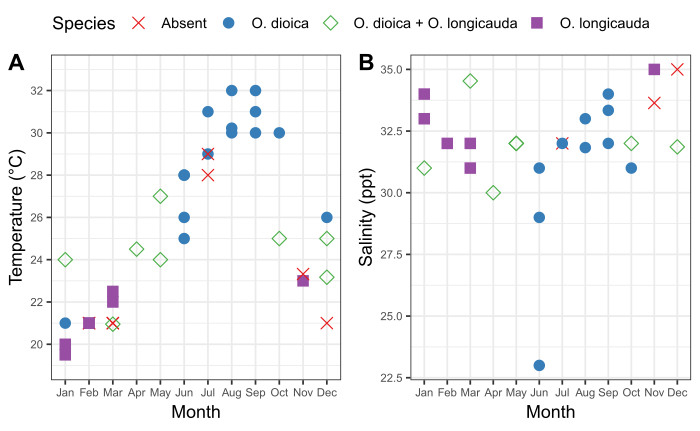
Figure 6: Seasonality of O. dioica in Okinawa.
Presence and absence of O. dioica and O. longicauda in relation to seasonal changes in (A) temperature and (B) salinity at harbors in Ishikawa (26°25'39.3"N 127°49'56.6"E) and Kin (26°26'40.2"N 127°55'00.3"E) between 2015-2019. Each species was recorded as present if more than 50 animals were manually counted. Temperature and salinity measurements of surface water were recorded. Please click here to view a larger version of this figure.
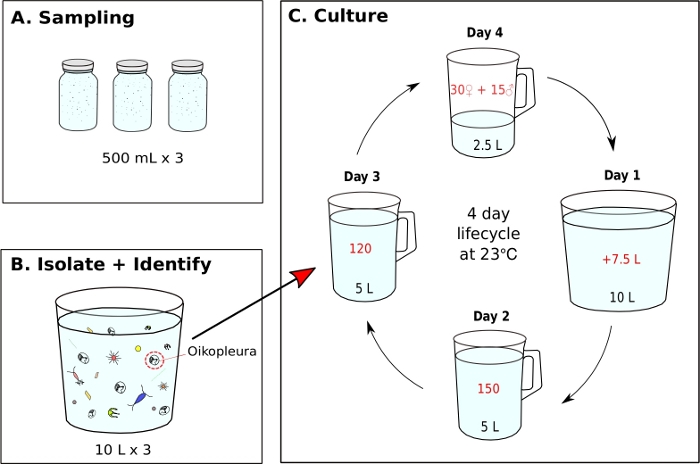
Figure 7: Flow chart for initiating O. dioica monoculture.
(A) Three, 500 mL plankton samples are collected from a sampling site (B) Each sample jar is diluted and O. dioica is isolated from the rest of plankton (C) A monoculture of O. dioica is initiated by manually transferring 120 Day 3 animals to a new beaker containing 5 L of fresh filtered seawater (fSW). Set up a spawning beaker containing 30 females, 15 males and 2.5 L of fresh fSW. The first morning post-spawning (Day1), carefully empty the spawning beaker with the new generation of animals into a beaker containing 7.5 L of fresh fSW. On the second day post-spawning (Day 2), transfer 150 animals into a beaker containing 5 L fresh fSW. On the third day post-spawning (Day 3), transfer 120 animals into a beaker containing 5 L fresh fSW. On the final day (Day 4), set up a new spawning beaker containing 30 females, 15 males and 2.5 L fresh fSW in preparation of the next generation. The animals have a 4-day lifecycle at 23 °C. Please click here to view a larger version of this figure.
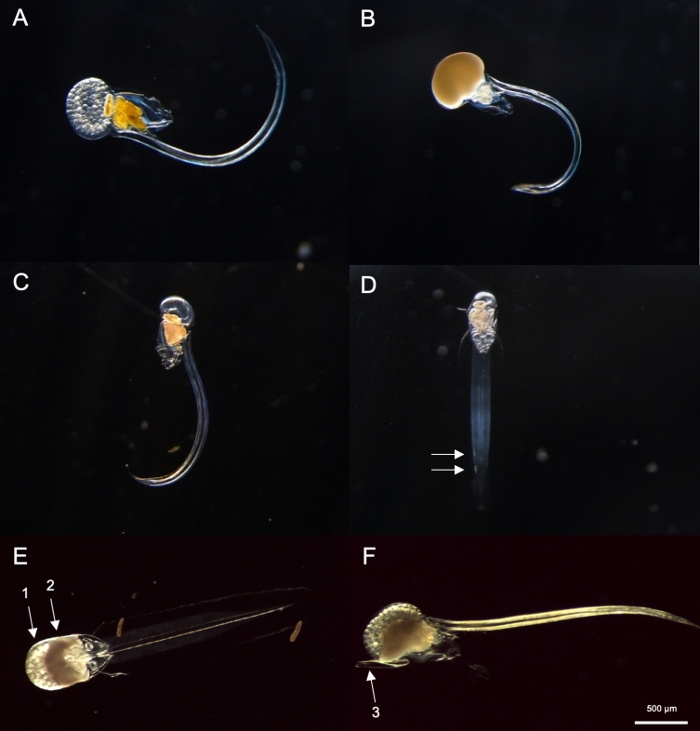
Figure 8: Identification of Oikopleura spp. (A-D: O. dioica, E and F: O. longicauda).
(A) Female O. dioica with eggs (B) Male O. dioica with sperm (C) Lateral view of immature O. dioica (D) Ventral view of immature O. dioica with two subchordal cells indicated with white arrows (E) Ventral view of mature O. longicauda carrying eggs (arrow 1) and sperm (arrow 2) (F) Lateral view of O. longicauda showing velum (arrow 3). Please click here to view a larger version of this figure.
| Reagents | Chemical products | Amount | Final vol. (mL) | Sterilization | Stock / Opened |
| Solution A | Na2EDTA | 45 g | 1000 | Autoclave | -20 °C / 4 °C |
| NaNO3 | 100 g | ||||
| H3BO3 | 33.6 g | ||||
| NaH2PO4 | 20 g | ||||
| MnCl2·4H2O | 0.36 g | ||||
| FeCl3·6H2O | 1.3 g | ||||
| Solution B | 1.0 mL | ||||
| Solution B | ZnCl2 | 2.1 g | 1000 | Autoclave | 4 °C / 4 °C |
| CoCl2·6H2O | 2.0 g | ||||
| (NH4)6Mo7O24·4H2O | 0.9 g | ||||
| CuSO4·5H2O | 2.0 g | ||||
| *HCl | — mL | ||||
| Vitamin | Thiamin (B1) ·HCl | 200 mg | 1000 | Autoclave | -20 °C / 4 °C |
| Biotin | 1 mg | ||||
| Cobalamin (B12) | 1 mg | ||||
| Sodium silicate | Na2SiO3 | 5% | 1000 | 0.22 µm filter | 4 °C / 4 °C |
| Streptomycin | C21H39N7O12 | 25 mg/mL | 50 | 0.22 µm filter | -20 °C / -20 °C |
Table 1: Recipe of reagents necessary for the maintenance of algal food. After dissolving all the chemical listed for solution B, HCl is added until the solution becomes clear with no turbidity. All the reagents are sterilized by either autoclaving (120 °C, 25 min) or by use of a 0.22 m filter. All the reagents except for the vitamin stocks are sterilized after the addition of specified chemical. For the vitamin stocks, autoclave the water first, and then dissolve the listed chemical. Storage temperatures for stock and opened reagents are listed.
| Culture type | Algal spp. | ASW (mL) | Vitamin | Solution A | Sodium silicate | Streptomycin | Algae (mL) / Culture type | Incubate / Store | Frequency |
| Stock culture | Chaeto | 60 | 1/2000 | 1/2000 | 1/4000 (Chaeto only) |
1/1000 (All except for Syn) |
0.03 / stock | 17°C / 4°C | Biweekly |
| Iso | 60 | 0.03 / stock | |||||||
| Rhino | 80 | 0.06 / stock | |||||||
| Syn | 60 | 0.03 / stock | |||||||
| Sub-culture | Chaeto | 500 | 1/2000 | 1/2000 | 1/4000 (Chaeto only) |
1/1000 (All except for Syn) |
10 / stock | 17°C / 17°C | Weekly |
| Iso | 500 | 10 / stock | |||||||
| Rhino | 500 | 20 / stock | |||||||
| Syn | 500 | 10 / stock | |||||||
| Working culture | Chaeto | 400 | 1/2000 | 1/2000 | 1/4000 (Chaeto only) |
1/1000 (All except for Syn) |
100 / sub | RM / RM | Every 4 days |
| Iso | 400 | 100 / sub | |||||||
| Rhino | 400 | 150 / sub | |||||||
| Syn | 400 | 100 / sub |
Table 2: Instruction for the maintenance of three algal culture types. Add the specified amount of supplements to flasks containing autoclaved seawater. Inoculate each flask with specified amount of algal culture. Incubate and store algal cultures at specified temperatures. Inoculate new stock culture and sub-culture from the previous stock culture, and new working culture from the previous sub-culture. Inoculate new stock culture, sub-culture, and working culture every two weeks, one week, and four days, respectively. This schedule provides enough food for approximately 10 beakers of O. dioica culture. Maintain 2 – 3 sets of each algal culture type as back-ups. RM – room temperature.
| Day | Algal spp. | 9AM and 5PM | 12PM |
| 1 | Chaeto | — | — |
| Iso | 1000 | 2000 | |
| Syn | 20,000 | 40,000 | |
| 2 | Chaeto | 1000 | 2000 |
| Iso | 2000 | 2000 | |
| Rhino | 1000 | 1000 | |
| 3 | Chaeto | 3000 | 4000 |
| Iso | 3000 | 4000 | |
| Rhino | 1500 | 1500 | |
| 4 | Chaeto | 1000 | 2000 |
| Iso | 1000 | 2000 | |
| Rhino | 1000 | 1000 |
Table 3: Algal concentration per feeding- modified from Bouquet et al.27. Algal concentrations (cells mL-1) and algal species used for daily feeding during the 4-day lifecycle of Okinawa O. dioica.
Supplemental File 1: Daily feeding chart. Daily feeding amounts for each culture beaker are automatically calculated after entering daily algal absorbance measurements (OD), the size of the animals (Day), and the volume of seawater (SW vol.) in each culture beaker. Growth curves of R. reticulata and Synechococcus sp. were adapted from Bouquet et al.27. Please click here to download this file.
Supplemental File 2: How to connect synchronous motor to acrylic paddle. Tightly screw on the paddle to the motor using a hexagon wrench. Please click here to download this file.
Discussion
To facilitate flexibility in the establishment of O. dioica culture, it is important to understand the animals’ natural habitat. Seasonal data provides information about the ranges of physical parameters, which can be used to guide laboratory culturing conditions. It also helps in understanding seasonal fluctuations in the abundance of animals. In Okinawa, O. dioica is most reliably found from June to October. However, in Tokyo bay, populations peak in February and October41. Although culturing of O. dioica is often reported at 20 °C or lower27,28,29, Okinawan O. dioica shows better survival at temperatures above 20 °C; this might be explained by the fact the minimum surface seawater temperature in Okinawa is ~20 °C (Figure 6). The abundance of O. dioica might also be influenced by phytoplankton blooms42 and predator abundance43,44. Regardless of where O. dioica are collected, understanding the seasonality of local populations maximizes the chance of sampling and culturing success.
Given the appropriate season and location, net sampling is an effective way of collecting large numbers of Oikopleura with minimal effort. Plankton nets with smaller mesh size (60-70 µm) may also be used to collect all stages of the animals. Fully mature animals are rarely found in the net, perhaps due to their fragility at the end of the lifecycle. Therefore, species identification followed by sampling is achieved by microscopic observation of subchordal cells. Mature individuals usually appear one or two days post-sampling as animals continue to grow in the laboratory. Although net sampling is efficient, alternative sampling methods might be necessary in different circumstances. For example, net sampling near urban areas can collect large numbers of phytoplankton, making it difficult to isolate Oikopleura. In such cases, simple bucket sampling to collect surface seawater or boat sampling from areas beyond the harbor are recommended. The results showed that the gradual change in salinity due to consecutive days of rain did not affect the abundance of O. dioica; however, shore sampling immediately after extreme weather events such as tropical cyclones should be avoided. These events cause sudden and drastic biogeochemical changes in a sheltered body of water45,46. The stormwater runoff may carry pollutants, sediments, and excess nutrients, which increase turbidity and lower water quality47. Filter-feeding plankton, such as Oikopleura, may be especially susceptible to these changes due to their mode of feeding and limited mobility. In such a circumstance, we recommend postponing sampling for a few days until the local conditions return to normal.
The introduction of a multi-step filter system is essential to maintain small, filter-feeding organisms such as O. dioica. Using poorly filtered seawater (for example, a 25 µm mesh in the previous culture system), the culture was often unstable especially during summer, potentially due to the higher abundance of phytoplankton. Although some phytoplankton are beneficial to O. dioica growth, others produce biotoxins that can cause abnormal development of O. dioica embryos48. In addition, a high concentration of diatoms such as Chaetoceros spp. are potentially harmful to O. dioica growth as they can possess long setae which can clog the house and prevent efficient feeding49. We frequently observed houses of small animals being clogged by C. calcitrans setae; therefore, we now feed C. calcitrans only to animals at Day 2 and older (Table 3).
Although it was not a problem here, small scale long-term culturing of O. dioica can experience sudden drops in population size due to a genetic bottleneck; in such cases, Martí-Solans et al.29 recommend adding new wild individuals to the culture every 20 generations.
The Oikopleura culture system is flexible. A stable culture can be established within a week. Long-term culturing of O. dioica is possible on a modest budget with non-specialist equipment. The daily effort required for the maintenance of 5-10 beakers of Oikopleura is generally less than 2 hours with 2 people. O. dioica can also be maintained in artificial seawater, which is beneficial to those without access to natural seawater28. Long-term storage of algal food is possible using solid-culture and cryopreservation29. Moreover, O. dioica sperm can be cryopreserved, and remain viable for more than a year50. All of these factors mean that cultures can be easily re-established. Finally, past experience with accidental culturing of Pleurobrachia sp. may suggest that the culturing system developed for Oikopleura could potentially be extended to a broader community of fragile pelagic organisms.
O. dioica continues to provide powerful insights into various biological fields. An understanding of local seasonality, a meticulous culture system, and a few dedicated individuals allow effective culture to be established with little effort. Oikopleura culture system provides the baseline resources to investigate a wide range of biological fields relating to ecology, development, genomics, and evolution of this unique marine chordate.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We are grateful to Garth Ilsley for his support in establishing the culture system. We acknowledge Ritsuko Suyama’s and Sylvain Guillot’s contributions to early sampling and species identification efforts. Special thanks are due to Hiroki Nishida, Takeshi Onuma, and Tatsuya Omotezako for their generous support and guidance throughout, including initial establishment of the local culturing system and sharing animals and microalgal culture. We also thank Daniel Chourrout, Jean-Marie Bouquet, Anne Aasjord, Cristian Cañestro, and Alfonso Ferrández-Roldán for sharing their expertise on sampling and culturing. Jai Denton, Charles Plessy, and Jeffrey Jolly provided invaluable feedback on the manuscript. Charlotte West formulated a generalized equation for algal calculation. Finally, we thank OIST for funding, Mary Collins and the OIST Fieldwork Safety Committee for advice on safe sampling procedures, the staff of OIST machine shop for the construction of culturing and sampling equipment, and Koichi Toda for delivering seawater.
Materials
| Activated charcoal | Sigma | C2764-2.5KG | |
| Alluminum pulley | Rainbow Products | 10604-10607 | |
| Biotin | Sigma | B4501-100MG | |
| Boric acid | Wako | 021-02195 | |
| Cobalamin (B12) | Sigma | V2876-100MG | |
| Cobalt(II) chloride hexahydrate | Wako | 036-03682 | |
| Copper(II) sulfate pentahydrate | Wako | 039-04412 | |
| Disodium edetate hydrate | Wako | 044-29525 | |
| Hexaammonium heptamolybdate tetrahydrate | Wako | 019-03212 | |
| Hexagon wrench | Anex | No.6600 | |
| Hydrochloric acid | Wako | 080-01066 | |
| Iron(III) chloride hexahydrate | Wako | 091-00872 | |
| Jebao programmable auto dosing pump | Jebao | DP-4 | |
| Magnet pump | REI-SEA | RMD-201 | |
| Manganese(II) chloride tetrahydrate | Wako | 134-15302 | |
| Polypropylene wound cartridge filter | Advantec | TCW-10N-PPS | |
| TCW-5N-PPS | |||
| TCW-1N-PPS | |||
| Screwless terminal block | SATO PARTS | SL4500 | |
| Simple plankton net | RIGO, Japan | 5512-C | |
| Sodium metasilicate | Sigma | 307815-1KG | |
| Sodium nitrate | Wako | 195-02545 | |
| Sodium phosphate monobasic anhydrous | MP Biomedicals | 194740 | |
| Streptomycin sulfate salt | Sigma | S6501-25G | |
| Synchronous electric motor | Servo | D5N6Z15M | |
| Thiamin hydrochloride | Wako | 201-00852 | |
| UV sterilizer | Iwaki | UVF-1000 | |
| Zinc chloride | MP Biomedicals | 194858 |
Riferimenti
- Travis, J. Is It What We Know or Who We Know? Choice of Organism and Robustness of Inference in Ecology and Evolutionary Biology (American Society of Naturalists Presidential Address). The American Naturalist. 167 (3), 303-314 (2006).
- Jenner, R. A., Wills, M. A. The choice of model organisms in evo-devo. Nature Reviews Genetics. 8 (4), 311-314 (2007).
- Irigoien, X., Huisman, J., Harris, R. P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature. 429 (6994), 863-867 (2004).
- Wilson, S., Ruhl, H., Smith, J. Zooplankton fecal pellet flux in the abyssal northeast Pacific: A 15 year time-series study. Limnology and Oceanography. 58 (3), 881-892 (2013).
- Steinberg, D. K., Lomas, M. W., Cope, J. S. Long-term increase in mesozooplankton biomass in the Sargasso Sea: Linkage to climate and implications for food web dynamics and biogeochemical cycling. Global Biogeochemical Cycles. 26 (1), 1004 (2012).
- Lombard, F., Kiørboe, T. Marine snow originating from appendicularian houses: Age-dependent settling characteristics. Deep Sea Research Part I: Oceanographic Research Papers. 57 (10), 1304-1313 (2010).
- Fenaux, R., Bone, Q. . The Biology of Pelagic Tunicates. , 251-264 (1998).
- Hopcroft, R. R., Gorsky, G., Youngbluth, M. J., Deibel, D. . Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularians. , 45-57 (2005).
- Walters, T. L., Gibson, D. M., Frischer, M. E. Cultivation of the Marine Pelagic Tunicate Dolioletta gegenbauri (Uljanin 1884) for Experimental Studies. Journal of Visualized Experiments. (150), e59832 (2019).
- Deibel, D. Feeding mechanism and house of the appendicularian Oikopleura vanhoeffeni. Marine Biology. 93 (3), 429-436 (1986).
- Spada, F., et al. Molecular patterning of the oikoplastic epithelium of the larvacean tunicate Oikopleura dioica. Journal of Biological Chemistry. 276 (23), 20624-20632 (2001).
- Flood, P. R., Gorsky, G., Youngbluth, M. J., Deibel, D. . Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularians. , 59-85 (2005).
- Tokioka, T. Studies on the distribution of appendicularians and some thaliaceans of the North Pacific, with some morphological notes. Publication of the Seto Marine Biological Laboratory. (8), 351-443 (1960).
- Alldredge, A. L. Discarded appendicularian houses as sources of food, surface habitats, and particulate organic matter in planktonic environments. Limnology and Oceanography. 21 (1), 14-24 (1976).
- Clarke, C., Roff, J. C. Abundance and biomass of herbivorous zooplankton off Kingston, Jamaica, with estimates of their annual production. Estuarine, Coastal and Shelf Science. 31 (4), 423-437 (1990).
- Hopcroft, R. R., Roff, J. C. Zooplankton growth rates: extraordinary production by the larvacean Oikopleura dioica in tropical waters. Journal of Plankton Research. 17 (2), 205-220 (1995).
- Hopcroft, R. R., Roff, J. C. Production of tropical larvaceans in Kingston Harbour, Jamaica: are we ignoring an important secondary producer. Journal of Plankton Research. 20 (3), 557-569 (1998).
- Mochioka, N., Iwamizu, M. Diet of anguilloid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Marine Biology. 125 (3), 447-452 (1996).
- Sakaguchi, S. O., et al. Morphological identity of a taxonomically unassigned cytochrome c oxidase subunit i sequence from stomach contents of juvenile chum salmon determined using polymerase chain reaction. Fisheries Science. 83 (5), 757-765 (2017).
- Fenaux, R., Bone, Q. . The Biology of Pelagic Tunicates. , 25-34 (1998).
- Sato, R., Tanaka, Y., Ishimaru, T. House production by Oikopleura dioica (Tunicata, Appendicularia) under laboratory conditions. Journal of Plankton Research. 23 (4), 415-423 (2001).
- Flood, R., Deibel, D., Bone, Q. . The Biology of Pelagic Tunicates. , 105-124 (1998).
- Alldredge, A., Gorsky, G., Youngbluth, M. J., Deibel, D. . Response of Marine Ecosystems to Global Change: Ecological Impact of Appendicularians. , 309-326 (2005).
- Katija, K., Sherlock, R. E., Sherman, A. D., Robison, B. H. New technology reveals the role of giant larvaceans in oceanic carbon cycling. Science Advances. 3 (5), 1602374 (2017).
- Katija, K., Choy, C. A., Sherlock, R. E., Sherman, A. D., Robison, B. H. From the surface to the seafloor: How giant larvaceans transport microplastics into the deep sea. Science Advances. 3 (8), 1700715 (2017).
- Hidaka, K. Species composition and horizontal distribution of the appendicularian community in waters adjacent to the Kuroshio in winter-early spring. Plankton and Benthos Research. 3 (3), 152-164 (2008).
- Bouquet, J. M., et al. Culture optimization for the emergent zooplanktonic model organism Oikopleura dioica. Journal of Plankton Research. 31 (4), 359-370 (2009).
- Nishida, H. Development of the appendicularian Oikopleura dioica: culture, genome, and cell lineages. Development, Growth & Differentiation. 50, 239-256 (2008).
- Martí-Solans, J., et al. Oikopleura dioica culturing made easy: A Low-Cost facility for an emerging animal model in Evo Devo. Genesis. 53 (1), 183-193 (2015).
- Holland, L. Z. Tunicates. Current Biology. 26 (4), 146-152 (2016).
- Delsuc, F., Brinkmann, H., Chourrout, D., Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 439 (7079), 965-968 (2006).
- Seo, H. C., et al. Miniature genome in the marine chordate Oikopleura dioica. Science. 294 (5551), 2506 (2001).
- Fredriksson, G., Olsson, R. The subchordal cells of Oikopleura dioica and O. albicans (Appendicularia, Chordata). Acta Zoologica. 72 (4), 251-256 (1991).
- Paffenhöfer, G. A. The cultivation of an appendicularian through numerous generations. Marine Biology. 22 (2), 183-185 (1973).
- Fenaux, R., Gorsky, G. Nouvelle technique d’élevage des appendiculaires. Rapports et Procés-Verbaux des Réunions-Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée. 29, 291-292 (1985).
- Fujii, S., Nishio, T., Nishida, H. Cleavage pattern, gastrulation, and neurulation in the appendicularian, Oikopleura dioica. Development Genes and Evolution. 218 (2), 69-79 (2008).
- Patry, W. L., Bubel, M., Hansen, C., Knowles, T. Diffusion tubes: a method for the mass culture of ctenophores and other pelagic marine invertebrates. PeerJ. 8, 8938 (2020).
- Fenaux, R. The classification of Appendicularia (Tunicata): history and current state. Memoires de I’Institut oceanographique. , (1993).
- Shiga, N., Chihara, M., Murano, M. . Illustrated Guide to Marine Plankton in Japan. , 1393-1414 (1997).
- Gorsky, G., Castellani, C., Castellani, C., Edwards, M. . Marine Plankton: A practical guide to ecology, methodology, and taxonomy. , 599-606 (2017).
- Sato, R., Ishibashi, Y., Tanaka, Y., Ishimaru, T., Dagg, M. J. Productivity and grazing impact of Oikopleura dioica (Tunicata, Appendicularia) in Tokyo Bay. Journal of Plankton Research. 30 (3), 299-309 (2008).
- Nakamura, Y., Suzuki, K., Suzuki, S. Y., Hiromi, J. Production of Oikopleura dioica (Appendicularia) following a picoplankton ‘bloom’in a eutrophic coastal area. Journal of Plankton Research. 19 (1), 113-124 (1997).
- Nakamura, Y. Blooms of tunicates Oikopleura spp. and Dolioletta gegenbauri in the Seto Inland Sea, Japan, during summer. Hydrobiologia. 385 (1-3), 183-192 (1998).
- Uye, S. I., Ichino, S. Seasonal variations in abundance, size composition, biomass and production rate of Oikopleura dioica (Fol)(Tunicata: Appendicularia) in a temperate eutrophic inlet. Journal of Experimental Marine Biology and Ecology. 189 (1-2), 1-11 (1995).
- Tsuchiya, K., et al. Phytoplankton community response and succession in relation to typhoon passages in the coastal waters of Japan. Journal of Plankton Research. 36 (2), 424-438 (2014).
- Lopez-Lopez, L., et al. Effects of typhoons on gelatinous carnivore zooplankton off Northern Taiwan. Cahiers de Biologie Marine. 53, 349-355 (2012).
- Ares, &. #. 1. 9. 3. ;., et al. Extreme storm-induced run-off causes rapid, context-dependent shifts in nearshore subtropical bacterial communities. bioRxiv. , (2019).
- Torres-Águila, N. P., et al. Diatom bloom-derived biotoxins cause aberrant development and gene expression in the appendicularian chordate Oikopleura dioica. Communications Biology. 1 (1), 1-11 (2018).
- Troedsson, C., Frischer, M. E., Nejstgaard, J. C., Thompson, E. M. Molecular quantification of differential ingestion and particle trapping rates by the appendicularian Oikopleura dioica as a function of prey size and shape. Limnology and Oceanography. 52 (1), 416-427 (2007).
- Ouchi, K., Nishino, A., Nishida, H. Simple procedure for sperm cryopreservation in the larvacean tunicate Oikopleura dioica. Zoological Science. 28 (1), 8-11 (2011).

