An Improved Protocol to Purify and Directly Mono-Biotinylate Recombinant BDNF in a Tube for Cellular Trafficking Studies in Neurons
Summary
Recombinant BDNF containing an Avi sequence (BDNFAvi) is produced in HEK293 cells in a cost-effective manner and is purified by affinity chromatography. BDNFavi is then directly mono-biotinylated with the enzyme BirA in a tube. BDNFavi and mono-biotinylated BDNFavi retain their biological activity when compared to commercially available BDNF.
Abstract
Recombinant BDNF containing an Avi sequence (BDNFAvi) is produced in HEK293 cells and then cost-effectively purified by affinity chromatography. A reproducible protocol was developed to directly mono-biotinylate BDNFAvi with the enzyme BirA in a tube. In this reaction, mono-biotinylated BDNFAvi retains its biological activity.
Neurotrophins are target-derived growth factors playing a role in neuronal development and maintenance. They require rapid transport mechanisms along the endocytic pathway to allow long-distance signaling between different neuronal compartments. The development of molecular tools to study the trafficking of neurotrophins has enabled the precise tracking of these proteins in the cell using in vivo recording. In this protocol, we developed an optimized and cost-effective procedure for the production of mono-biotinylated BDNF. A recombinant BDNF variant containing a biotinylable avi sequence (BDNFAvi) is produced in HEK293 cells in the microgram range and then purified in an easily scalable procedure using affinity chromatography. The purified BDNF can then be homogeneously mono-biotinylated by a direct in vitro reaction with the enzyme BirA in a tube. The biological activity of the mono-biotinylated BDNF (mbtBDNF) can be conjugated to streptavidin-conjugated to different fluorophores. BDNFAvi and mbtBDNF retain their biological activity demonstrated through the detection of downstream phosphorylated targets using western blot and activation of the transcription factor CREB, respectively. Using streptavidin-quantum dots, we were able to visualize mbtBDNF internalization concomitant with activation of CREB, which was detected with a phospho-CREB specific antibody. In addition, mbtBDNF conjugated to streptavidin-quantum dots was suitable for retrograde transport analysis in cortical neurons grown in microfluidic chambers. Thus, in tube produced mbtBDNF is a reliable tool to study physiological signaling endosome dynamics and trafficking in neurons.
Introduction
Neurons are the functional units of the nervous system possessing a complex and specialized morphology that allows synaptic communication, and thus, the generation of coordinated and complex behavior in response to diverse stimuli. Neuronal projections such as dendrites and axons are critical structural features involved in neuronal communication, and neurotrophins are crucial players in determining their morphology and function1. Neurotrophins are a family of secreted growth factors that include NGF, NT-3, NT-4, and brain-derived neurotrophic factor (BDNF)2. In the central nervous system (CNS), BDNF participates in diverse biological processes including neurotransmission, dendritic arborization, maturation of dendritic spines, long-term potentiation, among others3,4. Therefore, BDNF plays a critical role in regulating neuronal function.
Diverse cellular processes regulate BDNF dynamics and function. On the neuronal surface, BDNF binds the tropomyosin receptor kinase B (TrkB) and/or the p75 neurotrophin receptor (p75). BDNF-TrkB and BDNF-p75 complexes are endocytosed and sorted in different endocytic organelles5,6,7,8. Correct intracellular trafficking of the BDNF/TrkB complex is required for proper BDNF signaling in different neuronal circuits9,10,11. For this reason, a deep understanding of BDNF trafficking dynamics and its alterations found in pathophysiological processes is essential to understand BDNF signaling in health and disease. The development of novel and specific molecular tools to monitor this process will help to drive this field forward and allow a better grasp of the regulatory mechanisms involved.
There are several tools available for the study of BDNF trafficking in neurons. A commonly used methodology involves the transfection of recombinant BDNF tagged with fluorescent molecules such as green fluorescent protein (GFP) or the monomeric fluorescent red-shifted variant of GFP mCherry12,13. However, a major shortcoming of BDNF overexpression is that it eliminates the possibility of delivering known concentrations of this neurotrophin. Also, it may result in cellular toxicity, obscuring the interpretation of results14. An alternative strategy is the transfection of an epitope-tagged TrkB, such as Flag-TrkB. This methodology allows the study of TrkB internalization dynamics15, but it also involves transfection, which might result in altered TrkB function and cellular toxicity. To overcome these methodological hurdles, recombinant variants of NGF and BDNF containing an Avi sequence (BDNFAvi), which can be mono-biotinylated by the biotin-ligase enzyme BirA, were developed16,17. Biotinylated recombinant BDNF can be coupled to different streptavidin-bound tools, which include fluorophores, beads, paramagnetic nanoparticles among others for detection. In terms of live-cell imaging, quantum dots (QD) have become frequently used fluorophores, as they have desirable characteristics for single-particle tracking, such as increased brightness and resistance to photobleaching when compared to small molecule fluorophores18.
The production of mono-biotinylated BDNF (mbtBDNF) using BDNFAvi has been achieved by co-transfection of plasmids driving the expression of BDNFAvi and BirA, followed by the purification of the recombinant protein by affinity chromatography with a yield of 1-2 μg of BDNF per 20 mL of HEK293-conditioned culture media17. Here, we propose a modification of this protocol that allows for BDNFAvi purification from 500 mL of HEK293-conditioned media, which seeks to maximize protein recovery in a chromatography-column based protocol for ease of manipulation. The used transfection agent, polyethyleneimine (PEI), ensures a cost-effective method without sacrificing transfection yield. The mono-biotinylation step has been adapted to an in vitro reaction to avoid the complications associated with co-transfections and to ensure homogeneous labeling of BDNF. The biological activity of the mbtBDNF was demonstrated by western blot and fluorescence microscopy experiments, including activation of pCREB and live cell imaging to study retrograde axonal transport of BDNF in microfluidic chambers. The use of this protocol allows for optimized, high-yield production of homogenous mono-biotinylated and biologically active BDNF.
Protocol
All experiments were carried out in accordance with the approved guidelines of CONICYT (Chilean National Commission for Scientific and Technological Research). The protocols used in this study were approved by the Biosecurity and Bioethical and Animal Welfare Committees of the Pontificia Universidad Católica de Chile. Experiments involving vertebrates were approved by the Bioethical and Animal Welfare Committee of the Pontificia Universidad Católica de Chile.
NOTE: The following protocol was designed to purify BDNFAvi from a total volume of 500 mL of conditioned medium produced in HEK293 cells. The amount of conditioned medium that is produced and processed to purify BDNFAvi can be up or downscaled as needed. However, further optimization may be necessary under these circumstances. The composition of the culture media and buffers used throughout the protocol can be found in supplementary materials.
1. Production and purification of BDNFAvi from HEK293-conditioned media
- Transfection of HEK293 cells
- Grow HEK293 cells to 70% confluence in supplemented DMEM medium (10% bovine fetal serum, 1x glutamate supplement, 1x antibiotic/antimycotic) in 15 cm culture dishes at 37 ºC.
- Change the medium to transfection buffer.
- Prepare the PEI-DNA mixture for transfection. Use two different 15 mL conical tubes to dilute DNA and PEI 25 K, respectively. Dilute 20 μg of plasmid DNA in a final volume of 500 μL in one tube. Dilute 60 μg of linear PEI 25K in a final volume of 500 μL in the other tube. Incubate at room temperature for 5 min.
- Carefully pipette the DNA solution into the PEI tube, mixing once by up-down motion. Incubate at room temperature for 25 min.
- Drip 1 mL of the PEI-DNA mixture throughout each 15 cm dish. Incubate the cells with the PEI-DNA mixture for 3 h at 37 ºC.
- Change the medium to fresh incubation buffer.
- Media collection and storage
- Collect the medium from all the dishes 48 h after the transfection of HEK293 cells. Prepare concentrated stocks of the solutions described in the “supernatant modification buffer” section of Supplemental File 1 and add them to the HEK293 supernatant to achieve the listed final concentrations.
NOTE: Cells can be discarded or recovered for further analysis. - Incubate the medium in ice for 15 min.
- Aliquot the medium into centrifuge tubes.
- Centrifuge the medium at 10,000 x g for 45 min in a 4 °C centrifuge. This step allows the elimination of cell debris and dead cells suspended in the media.
- Collect the supernatants, add BSA at a final concentration of 0.1%. and then store at -20 °C. The media can be aliquoted before freezing for faster thawing during the purification step.
NOTE: Storage times of frozen conditioned media of up to 2 months have yielded positive results, longer storage times have not been evaluated.
- Collect the medium from all the dishes 48 h after the transfection of HEK293 cells. Prepare concentrated stocks of the solutions described in the “supernatant modification buffer” section of Supplemental File 1 and add them to the HEK293 supernatant to achieve the listed final concentrations.
- Media concentration and purification
- Thaw the media in a 37 °C thermoregulated bath.
- Aliquot the media into centrifuge tubes.
- Centrifuge the medium for 1 h at 3,500 x g in a 4 °C cooled centrifuge. This step allows the elimination of remaining cell debris to ensure adequate flow through the chromatography column.
- Use the protein concentrators with a 10 kDa cutoff to reduce the media from 500 mL to 100 mL. Follow the manufacturer’s recommended centrifugation parameters for optimal concentration.
- Add 500 μL of Ni-NTA agarose beads to the concentrated media and incubate overnight at 4 °C in a rocker.
- Assemble the chromatography apparatus and pour the media into it. Let it rest for 5 min and then open the 2-way stopcock to let the medium flow through.
- Wash the beads with 5 mL of wash buffer for 5 min. Make sure to resuspend the beads in the column. Drain the wash buffer by opening the 2-way stopcock. Repeat 3 times.
- Add 1 mL of elution buffer to the column. Make sure to resuspend the beads in the column. Incubate for 15 min, and then collect the eluate in a 1.5 mL microcentrifuge tube. Repeat this step 3 times for complete elution of BDNFAvi.
- Load 5 μL of each eluate and different concentrations of commercially available BDNF (40-160 ng) in a 15% polyacrylamide gel. Detect the purified protein by western blotting using an anti-BDNF antibody.
- Determine the concentration of the purified BDNFAvi in each eluate using the concentration curve prepared with the commercially available BDNF.
- Aliquot and store the purified BDNFAvi at -80 °C.
2. In vitro mono-biotinylation of BDNFAvi using the BirA enzyme
- In vitro mono-biotinylation reaction
- Prepare concentrated stock solutions of the biotinylation buffer reagents. The use of concentrated stocks will minimize the dilution of the recombinant protein.
- Take an aliquot of 800 ng of BDNFAvi and add the biotinylation buffer reagents and the enzyme BirA in a 1:1 molar relation to BDNF. For example, for a 200 μL final reaction volume add; 100 μL of solution containing 800 ng of BDNFAvi, 20 μL Bicine 0.5 M pH 8.3, 20 μL ATP 100 mM, 20 μL MgOAc 100 mM, 20 μL d-biotin 500 μM, 0.8-1 μg to 1 μL of BirA-GST, and complete to 200 μL with ultrapure water.
NOTE: Successful biotinylation reactions have been performed with aliquots of 400 μL containing a concentration of about 30 ng/ μL BDNFAvi, resulting in a homogeneously biotinylated BDNFAvi to a final concentration of ~20 ng/ μL in the final reaction. - Incubate the mixture at 30 °C in a hybridization oven for 1 h. Mix the content by tube inversion every 15 min.
- Add the same volume of ATP and BirA as in step 2.1.2 and repeat step 2.1.3.
- Store at -80 °C for future analyses or keep on ice for immediate use (e.g., biotinylation quality control).
- Biotinylation analysis
- Block 30 μL of streptavidin magnetic beads per BDNF sample in 1 mL of blocking buffer. Incubate at room temperature for 1 h in a microcentrifuge tube rotator.
- Precipitate the magnetic beads using a magnetic separation rack for 3 to 5 minutes or until the buffer appears completely cleared of the beads and discard the blocking buffer.
- Add 50 μL of fresh blocking buffer and 80 ng of mono-biotinylated BDNFAvi (mbtBDNF) sample to the beads, making sure to resuspend them completely by pippeting.
- Incubate at 4 °C for 1 h in a microcentrifuge tube rotator spinning at approximately 20 RPM.
- Collect the beads using the magnetic separation rack for 3 to 5 minutes, and collect the supernatant, keeping a 30 μL aliquot for analysis.
- Wash the beads one time with 500 μL of PBS, and then collect them using the magnetic separation rack for 3 to 5 minutes. Recover the supernatant and keep a 30 μL aliquot for analysis.
- Add 10 μL of 4x loading buffer to the beads.
- Heat the samples to 97 °C for 7 min to elute the mbtBDNF.
- Detect mbtBDNF using an anti-BDNF specific antibody19.
3. Verification of mbtBDNF biological activity
- Detection of pTrkB and pERK by western blot.
- Seed 2 million rat cortical neurons in 60 mm culture dishes.
- Culture the neurons for 7 days (DIV7). Then, change the medium to non-supplemented neurobasal mediun when starting the experiment.
- One hour after medium change, add mbtBDNF to a final concentration of 50 ng/mL. Incubate for 30 min at 37 ºC. Keep a negative control dish (non-stimulated with BDNF) and a positive control dish (treated with 50 ng/mL of commercially available BDNF).
- Collect the medium and gently wash every dish with 1x PBS. Collect and discard the 1x PBS.
- Place the dishes on ice and add 50-80 μL of lysis buffer to each dish. Use a cell scraper to lyse the cells.
NOTE: The lysis step should be performed as quickly as possible to avoid protein dephosphorylation and degradation. 1-2 minutes of vigorous scraping are enough to visualize the proteins of interest by western blotting. - Collect the lysis buffer and stir in a vortex mixer at highest speed for 5 s.
- Centrifuge the lysis buffer at 14,000 x g (4 °C) for 10 min. Collect the supernatant.
- Quantify the protein content of the supernatant by BCA protein quantification protocol20.
- Add loading buffer to an aliquot containing 30-50 μg of protein per condition and load it in a 12% polyacrylamide gel for western blotting. Detect pTrkB and pERK using specific phospho-antibodies to verify BDNFAvi biological activity.
- Verification of BDNF-QD biological activity by pCREB immunofluorescence.
- Seed 40,000 rat cortical neurons in 10 mm coverslips, previously autoclaved and treated with poly-L-lysine as described previously21.
- Culture the neurons for 7-8 days in neuronal maintenance buffer (see supplemental materials) at 37 ºC.
- To start the experiment, change the medium to unsupplemented neurobasal medium and incubate at 37 ºC for 1 h.
- Prepare mbtBDNF conjugated to quantum dots (BDNF-QD) by adding to a mbtBDNF aliquot, the necessary volume of quantum dot streptavidin conjugate (streptavidin-QD) to achieve a 1:1 BDNF-QD molar ratio. Then, dilute to 20 μL with neurobasal medium. Wrap the tube in aluminum foil to protect it from the light.
NOTE: Prepare another tube with the same volume of quantum dot streptavidin conjugate and dilute it to 20 μL with neurobasal medium as a negative control. - Incubate the mbtBDNF/ streptavidin-QD mixture for 30 min at room temperature in a rocker.
- Dilute the BDNF-QD to the desired final concentration (200 pM and 2 nM) in neurobasal medium.
- After 1 h of incubation with non-supplemented neurobasal medium, stimulate the neurons with BDNF-QD or streptavidin-QD (control) to a final concentration of 200 pM and 2 nM of BDNF for 30 min at 37 °C.
- Wash the coverslips 3 times with 1x PBS (37 °C) and fix the cells for 15 min by treating the coverslip with 4% paraformaldehyde solution containing phosphatase inhibitors.
- Wash the cells 3 times with PBS, and then incubate with blocking/permeabilization buffer (BSA 5%, Triton X-100 0.5%, 1x phosphatase inhibitor) for 1 h.
- Incubate with anti-pCREB antibody 1:500 (in 3% BSA, 0.1% Triton X-100) overnight at 4 °C.
- The following day, wash 3 times with 1x PBS, and incubate for 1 h with the secondary antibody 1:500 (3% BSA, 0.1% Triton X-100).
- Wash 3 times with 1x PBS. Add Hoechst nuclear stain solution (5 μg/mL) for 7 min.
- Wash 3 times with 1x PBS and mount.
- Visualization of retrograde axonal transport of BDNF-QD in live neurons
- Prepare microfluidic chambers and seed neurons as described previously16.
- After 7-8 days in culture, change the medium to non-supplemented neurobasal medium.
- Prepare mbtBDNF conjugated to quantum dots (BDNF-QD) by adding to a mbtBDNF aliquot, the necessary volume of quantum dot streptavidin conjugate (streptavidin-QD) to achieve a 1:1 BDNF-QD molar ratio. Then, dilute to 20 μL with neurobasal medium. Wrap the tube in aluminum foil to protect it from the light.
NOTE: Prepare another tube with the same volume of quantum dot streptavidin conjugate and dilute it to 20 μL with neurobasal medium as a control. - Incubate the mbtBDNF/ streptavidin-QD mixture for 30 min at room temperature in a rocker.
- Dilute the BDNF-QD to the desired final concentration (2 nM).
- After 1 h of incubation with non-supplemented neurobasal medium add the BDNF-QD or the control mixture to the axonal compartments of the microfluidic chamber. Incubate for 210 min at 37 ˚C to ensure a net retrograde transport of BDNF-QD.
- For live-cell imaging, visualize axonal retrograde transport in the segment of the microgrooves that is proximal to the cell body compartment using a 100x objective using a microscope suitable for these purpose (37 °C and 5% CO2). Acquire images at 1 frame/s.
Representative Results
The use of a chromatographic column-based protocol allows the processing of significant volumes of HEK293 conditioned media. In Figure 1, the results of the purification of BDNFAvi from 500 mL of conditioned media are shown. Consecutive elutions of BDNFAvi from the Ni-NTA agarose beads yield decreasing concentrations of BDNFAvi (Figure 1A). After four consecutive elutions (each lasting 15 min), the majority of the BDNF captured by the beads is recovered. The concentrations of the eluates range from 6 to 28 ng/μL, and the total yield amounted to approximately 60 μg of BDNFAvi (Table 1). The produced BDNFAvi was then efficiently biotinylated by an in vitro reaction mediated by BirA-GST, as demonstrated by the lack of non-biotinylated BDNFAvi in the supernatant (Figure 1B). Please note that the biotinylation presented in Figure 1B corresponds to an aliquot of the total BDNF produced, but the reaction can be scaled up for bigger volumes.
Then, the biological activity of mbtBDNF was evaluated using 2 different experimental approaches. First, cortical neurons seeded in 60 mm plates (2 million neurons, DIV7) were stimulated with 50 ng/mL of mbtBDNF for 30 min, and then proteins were prepared for western blot analysis. The biological activity of the mbtBDNF was quantified by detecting pTrkB (Y515) and pERK (T202/Y204). Binding of BDNF to TrkB triggers the activation of the receptor through an autophosphorylation reaction in its intracellular domain, and ERK is a known target of the BDNF signaling pathway22. The bands for both phosphorylated proteins had a similar intensity in neurons treated with commercial BDNF and mbtBDNF, and both showed a stronger signal than control condition (Figure 2A). Then, the biological activity of mbtBDNF coupled to streptavidin-QD was evaluated to demonstrate that they can be used in live imaging experiments. Cortical neurons were seeded in 10 mm covers (40,000 cells per cover, DIV7) and treated with a final concentration of 200 pM or 2 nM BDNF-QD for 30 min before fixing and staining for pCREB. CREB is a transcription factor which is targeted by activated ERK1/2 in cortical neurons22,23. Stimulating neurons with increasing concentrations of BDNF-QD resulted in a dose-dependent increase of phosphorylation of CREB and presence of QD particles surrounding the nucleus (Figure 2B), indicating that the BDNF-QD particles were endocytosed and triggered the activation of signaling pathways associated with BDNF-mediated TrkB activation. A twofold increase in pCREB signal was detected when stimulating neurons with a low concentration of BDNF-QD (200 pM), whereas stimulating with 2 nM resulted in a 3.5-fold increase in the pCREB signal (Figure 2C). These results demonstrate that the biotinylated BDNFAvi is biologically active, and that it does not lose its activity when coupled to streptavidin-QD, making it suitable for immunofluorescence and live cell imaging.
Finally, the imaging potential of BDNF-QD was evaluated in compartmentalized cultures using microfluidic chambers. Cortical neurons were seeded in microfluidic chambers (15 mm covers, 50,000 neurons per microfluidic chamber, DIV7) to separate the axonal and somatodendritic compartments and were stimulated with 2 nM BDNF-QD for 3.5 h. Live cell microscopy was performed, and the resulting kymographs were used to quantify the speed of BDNF-QD containing organelles (Figure 3A). An average moving speed of 0.91 μm/s was detected (Figure 3B), which is in line with previous analyses of cytoplasmic dynein-mediated transport7,16. Microfluidic chambers treated with 2 nM streptavidin-QD did not show moving QDs in the microgrooves, as shown by the kymograph (Figure 3A). Cells grown under the same conditions were stimulated with 500 pM or 2 nM BDNF-QD for 210 min, and then fixed and labelled with a nuclear staining. As shown in Figure 3C, neurons show a dose-dependent accumulation of BDNF-QD in all the analyzed sub-compartments, including the proximal and distal portions of the microgroove and the somatodendritic compartment. In contrast, control neurons showed almost no QD signal throughout the chamber. Therefore, the BDNF-QD can be detected in live and fixed cells in microfluidic chambers.
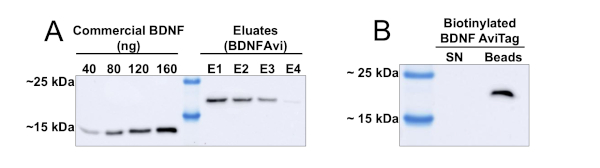
Figure 1: Production and mono-biotinylation of BDNFAvi in HEK293 cells. HEK293 cells were transfected using the PEI reagent and a BDNFAvi encoding plasmid and the conditioned media was collected after 48 h. BDNFAvi contains a 6x Histidine tag allowing purification using nickel-nitrilotriacetic acid (Ni-NTA) chromatography. Commercially available recombinant human BDNF has an expected molecular weight of ~13 kDa, whereas BDNFAvi displays a molecular weight of ~18 kDa. BDNFAvi bound to the resin was fully eluted with four consecutive elution steps. (A) Western blot using anti-BDNF antibodies to detect in house prepared recombinant BDNF and commercial BDNF. Aliquots containing known amounts of commercially available human BDNF and 5 μL of each eluate were loaded into an SDS-PAGE gel for detection of BDNFAvi using an antibody against BDNF. Table 1 indicates the concentrations of BDNFAvi present in each eluate. The amount and concentration of BDNF in each eluate was obtained by densitometric analysis and interpolation from the concentration curve of commercially available BDNF. (B) Verification of BDNFAvi biotinylation. Eighty nanograms of biotinylated BDNFAvi (mbtBDNF) were incubated with 30 μL of streptavidin coupled to magnetic beads (20% slurry) for 1 hr at 4 °C. Then, magnetic beads were isolated using a magnetic separator. The streptavidin beads were heated with loading buffer to elute the biotinylated BDNFAvi (beads lane). The supernatant (SN lane) was also treated with loading buffer, heated and loaded in the gel (SN lane). Please click here to view a larger version of this figure.
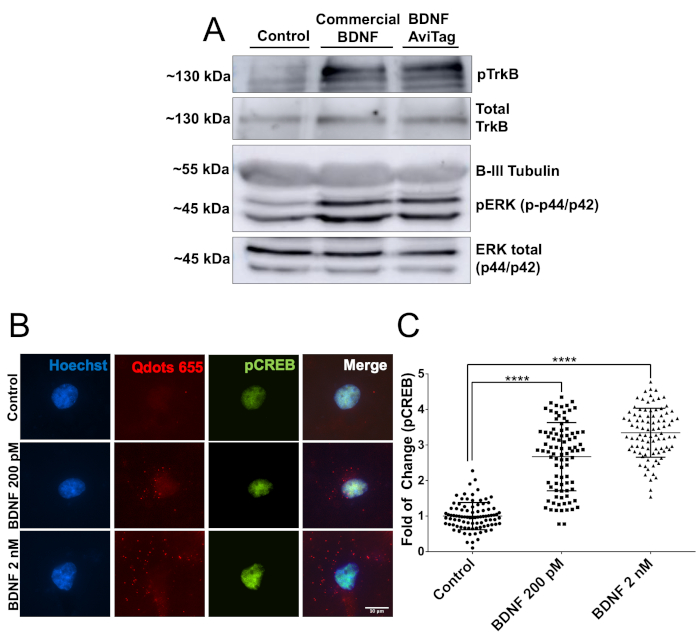
Figure 2: Verification of mbtBDNF biological activity. (A) DIV7 cortical neurons were serum starved for 1 h, and then stimulated with 50 ng/mL of commercially-available BDNF or mbtBDNF for 30 min. Proteins were extracted and loaded in an SDS-PAGE gel for analysis of TrkB and ERK1/2 phosphorylation using phospho-specific antibodies and compared to the total levels of the protein using antibodies against total TrkB and ERK1/2. (B) DIV7 cortical neurons were serum starved for 1 h, and then stimulated with a final concentration of 200 pM or 2 nM of mbtBDNF coupled to streptavidin-QD (BDNF-QD) for 30 min. Then, cells were fixed and pCREB was labelled for fluorescence microscopy analysis. (C) Quantification of nuclear pCREB fluorescence intensity. The results correspond to 90 neurons pooled together from 3 independent experiments, shown as mean ± SEM. The statistical analysis corresponds to a one-way ANOVA with Tukey’s multiple comparisons test (****p < 0.0001). Please click here to view a larger version of this figure.
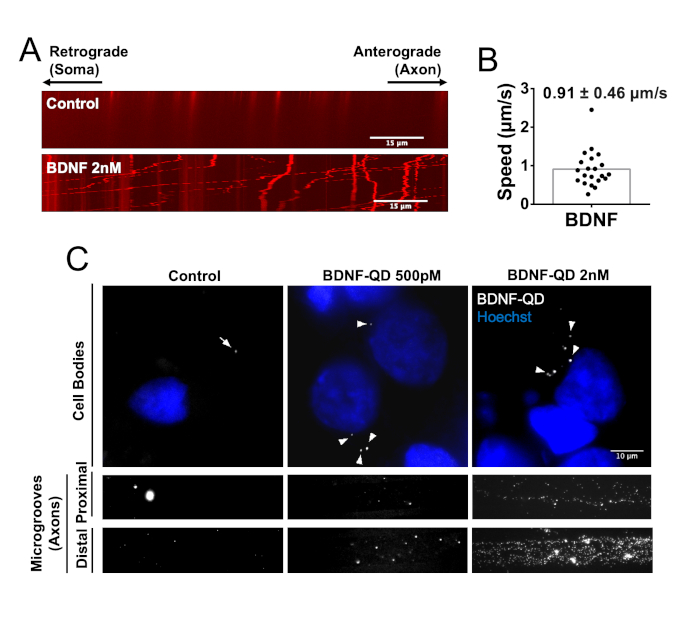
Figure 3: Visualization of BDNF-QD in live and fixed cells. (A) DIV7 cortical neurons grown in microfluidic chambers were stimulated in the axonal compartment with a final concentration of 2 nM BDNF-QD for 3.5 hrs, and then the proximal portion of the microgrooves was imaged using a live cell microscopy setting. Representative kymographs for control condition (treated with streptavidin-QD) and upon treatment with BDNF-QD are shown. (B) Quantification of the speed of moving BDNF-QD. Mobile puncta were defined as those that moved more than 10 μm in the 120 s of recording. (C) DIV7 cortical neurons grown in microfluidic chambers were stimulated in the axonal compartment with a final concentration of BDNF-QD of 500 pM or 2 nM for 3.5 hrs, and then fixed and labelled with Hoechst to visualize the nuclei. Representative images of the somatodendritic compartment and the distal and proximal portions of the microgrooves are shown. Please click here to view a larger version of this figure.
| Eluate | BDNF (ng) in 5μl | [ng/μl] | Total BDNF [μg] |
| E1 | 142.2 | 28.4 | 28.4 |
| E2 | 101.2 | 20.2 | 20.2 |
| E3 | 65.6 | 13.1 | 13.1 |
| E4 | 30.4 | 6.1 | 6.1 |
| 67.8 |
Table 1: Quantification of BDNFAvi purification yield (related to Fig. 1A). HEK293 cells were transfected with a plasmid driving BDNFAvi expression, and the protein was purified by Ni-NTA affinity chromatography. Protein concentration and final yield was calculated by densitometric analysis and interpolation in the known concentration curve of commercially available recombinant human BDNF.
Supplemental File 1: Culture media and buffer components Please click here to download this file.
Discussion
In this article, an optimized methodology for the production and purification of mbtBDNF in an affinity chromatography-based procedure is described, based on the work of Sung and collaborators17. The optimizations include the use of a cost-effective transfection reagent (PEI) while maintaining the efficiency of more expensive transfection methods such as lipofectamine. This optimization translates into a significant cost reduction in the protocol, allowing for scalability while maintaining high cost-effectiveness. The protocol also includes ease of use considerations, including the freezing of conditioned media for up to 2 months. These optimizations make the procedure adaptable to each laboratory’s needs, improve cost-effectiveness, and yield homogeneous and biologically active recombinant BDNF. The protocol can also be adapted to smaller scale productions by replacing the use of the chromatography apparatus with gravitational precipitation of the beads in conical tubes. This constitutes a viable methodology, but its less time-efficient and has resulted in lower yields in our experience. The biotin-labeled BDNF can then be coupled to different streptavidin-bound probes, including fluorophores and paramagnetic nanoparticles, making it a valuable tool to perform diverse types of experiments for the analysis of BDNF post-endocytic trafficking. Therefore, an optimized and simple production protocol for this protein is highly useful to laboratories working in this field.
Production of recombinant proteins with complex post-translational modifications, such as BDNF24, in prokaryotic systems often results in proteins that are not correctly folded and thus have poor biological activity25. Therefore, expression in mammalian cells is necessary to obtain a bioactive protein. The use of PEI has been described previously as a viable alternative for large-scale production of recombinant proteins in transfected mammalian cells25,26, and its efficiency in the transfection of the HEK293 cells in the context of academic laboratories has been highlighted27. Therefore, the use of this cell line represents a valid option to produce BDNFAvi on a scale that can be managed by an academic laboratory. The proposed protocol could be optimized further by the generation of a HEK293 cell line stably transfected with BDNFAvi, which would eliminate the transient transfection step, thus saving time and resources. Another potential source of optimization is the use of cells in suspension instead of adherent cells. HEK293 cells can be maintained in suspension, generating significant amounts of recombinant protein in the range of grams per liter28.
Another improvement in the protocol is the biotinylation of the BDNFAvi protein using an in vitro strategy, replacing the previous in vivo co-transfection protocol. Transient co-transfection can have unexpected results in terms of the expression of the constructs, as has been demonstrated in multiple cell lines and with several transfection reagents29. Various factors can affect the expression of transfected proteins in a co-transfection context, including vectors, cell types and plasmid concentration. This multiplicity of factors makes optimization and reproducibility a complex task. On the other hand, an in vitro methodology allows for better control over the conditions in which the biotinylation reaction takes place. This methodology results in reproducible and homogeneous labeling of recombinant BDNF.
As demonstrated by the biological activity verification experiments, the mbtBDNF produced using this protocol is comparable to commercially-available recombinant human BDNF in terms of BDNF-TrkB signaling pathway activation. The data also shows that coupling BDNF to streptavidin-QD does not interfere with BDNF-TrkB signaling. In addition, we showed that BDNF-QD can be detected by epifluorescence microscopy in live and fixed cells. Therefore, mbtBDNF represents a valuable tool for studying retrograde axonal trafficking and it presents significant advantages over alternative probes, such as BDNF-GFP16.The protocol described in this article provides a reliable and consistent methodology for the production of mbtBDNF, which can then be used in post-endocytic dynamics studies in different neuronal models expressing TrkB or p75. BDNF signaling has potent effects on neuronal morphology and function3,4,21, and has been recently proposed as a potential therapeutic tool to enhance neuronal regeneration30,31, making its study relevant in the fields of cellular biology and biomedicine. The study of the effects of BDNF signaling and trafficking will further advance our understanding of neuronal cell biology and may allow for the harnessing of its regenerative potential in clinical settings.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge financial support from Fondecyt (1171137) (FCB), the Basal Center of Excellence in Science and Technology (AFB 170005) (FCB), Millenium-Nucleus (P07/011-F) (FCB), the Wellcome Trust Senior Investigator Award (107116/Z/15/Z) (GS) and a UK Dementia Research Institute Foundation award (GS). This work was supported by the Unidad de Microscopía Avanzada UC (UMA UC).
Materials
| 2 way stopcock | BioRad | 7328102 | Chromatography apparatus component |
| 2-mercaptoethanol | Sigma | M6250 | BDNF elution buffer |
| Acrylamide/Bisacrylamide | BioRad | 1610154 | SDS-PAGE gel preparation |
| Amicon Ultra-15 10K | Millipore | UFC901024 | BDNF concentration |
| Ammonium Persulfate | Sigma | A9164 | SDS-PAGE gel preparation |
| anti B-III-Tubulin antibody | Sigma | T8578 | Western blot assays for BDNF biological activity detection |
| anti BDNF antibody | Alomone | AGP-021 | Western blot assays for BDNF quantification |
| anti BDNF antibody | Alomone | ANT-010 | Western blot assays for BDNF quantification |
| Anti ERK antibody | Cell Signaling | 9102 | Western blot assays for BDNF biological activity detection |
| anti pCREB antibody (S133) | Cell Signaling | 9198 | Western blot assays for BDNF biological activity detection |
| anti pERK antibody (T202, Y204) | Cell Signaling | 4370 | Western blot assays for BDNF biological activity detection |
| anti pTrkB antibody (Y515) | Abcam | ab109684 | Western blot assays for BDNF biological activity detection |
| Antibiotic/Antimycotic | Gibco | 15240-062 | HEK293 maintenance |
| ATP | Sigma | A26209 | BDNF monobiotinylation buffer |
| B-27 Supplement | Gibco | 17504-044 | Neuron maintenance |
| Bicine | Sigma | B3876 | BDNF monobiotinylation buffer |
| BirA-GST | BPS Bioscience | 70031 | Enzyme for BDNF AviTag monobiotinylation |
| Bovine Fetal Serum | HyClone | HC.SH30396.02 | HEK293 maintenance |
| Bovine Serum Albumin | Jackson ImmunoResearch | 001-000-162 | BDNF buffer modification component, blocking buffer for western blot and immunofluorescence |
| D-Biotin | Sigma | B4639 | BDNF monobiotinylation buffer |
| Dithiothreitol | Invitrogen | 15508-013 | |
| DMEM High Glucose Medium | Gibco | 11965-092 | Neuron seeding |
| DMEM Medium | Gibco | 11995-081 | HEK293 maintenance |
| Econo Column Funnel | BioRad | 7310003 | Chromatography apparatus component |
| EDTA | Merck | 108418 | |
| EZ-ECL Kit | Biological Industries | 1633664 | Protein detection by western blotting |
| Glutamax | Gibco | 35050-061 | Neuron and HEK293 maintenance |
| Glycerol | Merck | 104094 | BDNF elution buffer, lysis buffer for western blot assays |
| Hettich Rotina 46R Centrifuge | Hettich | Discontinued | Centrifuge used for clearing the medium of debris |
| Hettich Universal 32R Centrifuge | Hettich | Discontinued | Centrifuge used for protein concentrator centrifugation |
| Horse Serum | Gibco | 16050-122 | Neuron seeding |
| ImageQuant LAS 500 | GE Healthcare Life Sciences | 29005063 | Western blot image acquisition |
| Imidazole | Sigma | I55513 | BDNF buffer modification component |
| KCl | Winkler | BM-1370 | PBS component |
| KH2PO4 | Merck | 104873 | PBS component |
| Laminin | Invitrogen | 23017-015 | Cover coating for compartmentalized neurons |
| Luer Tubing Adaptor | BioRad | 7323245 | Chromatography apparatus component |
| Luminata™ Forte Western HRP Substrate | Millipore | WBLUF0100 | Protein detection by western blotting |
| Mg(CH3COO)2 | Merck | 105819 | BDNF monobiotinylation buffer |
| Mowiol 4-88 | Calbiochem | 475904 | Mounting reagent for immunofluorescence assays |
| MyOne C1 Streptavidin Magnetic Beads | Invitrogen | 65001 | Biotinylation verification |
| Na2HPO4 | Merck | 106586 | BDNF buffer modification component |
| NaCl | Winkler | BM-1630 | PBS component, BDNF buffer modification component |
| NaH2PO4 | Merck | 106346 | BDNF buffer modification component |
| Neurobasal Medium | Gibco | 21103-049 | Neuron maintenance |
| Ni-NTA Agarose Beads | Qiagen | 30210 | BDNF AviTag purification |
| Nikon Ti2-E | Nikon | Microscope for fluorescence imaging | |
| Nitrocellulose Membrane | BioRad | 1620115 | Protein transfer for western blotting |
| ORCA-Flash4.0 V3 Digital CMOS camera | Hamamatsu | C13440-20CU | Camera for epifluorescence imaging |
| P8340 Protease Inhibitor Cocktail | Sigma | P8340 | BDNF buffer modification component |
| Paraformaldehyde | Merck | 104005 | Fixative for immunofluorescence assays |
| Penicillin/Streptomycin | Gibco | 15140-122 | Neuron maintenance |
| Poli-D-Lysine | Corning | DLW354210 | Cover coating for compartmentalized neurons |
| Poli-L-Lysine | Millipore | P2363 | Cover coating for non-compartmentalized neurons |
| Poly-Prep Chromatography Column | BioRad | 7311550 | Chromatography apparatus component |
| Polyethyleneimine 25K | Polysciences Inc. | PLY-0296 | HEK293 transfection |
| Quantum Dots 655 streptavidin conjugate | Invitrogen | Q10121MP | Monobiotinylated BDNF AviTag label for live and fixed cell experiments |
| Saponin | Sigma | S4521 | Detergent for immunofluorescence assays |
| Sucrose | Merck | 107687 | |
| Syldgard 184 silicone elastomer base | Poirot | 4019862 | Microfluidic chamber preparation |
| TEMED | Sigma | T9281 | SDS-PAGE gel preparation |
| Tris | Winkler | BM-2000 | Lysis buffer component |
| Triton X100 | Merck | 108603 | Cell permeabilization in immunofluorescence and western blot assays |
| Trypsin-EDTA 0.5% | Gibco | 15400-054 | HEK293 passaging |
Riferimenti
- Huang, E., Reichardt, L. Neurotrophins: Roles in Neuronal Development and Function. Annual Review of Neuroscience. 24, 677-736 (2001).
- Skaper, S. D. The neurotrophin family of neurotrophic factors: an overview. Methods in Mollecular Biology. 846, 1-12 (2012).
- Gonzalez, A., Moya-Alvarado, G., Gonzalez-Billault, C., Bronfman, F. C. Cellular and molecular mechanism regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton. 73 (10), 612-628 (2016).
- Cunha, C., Brambilla, R., Thomas, K. A simple role for BDNF in learning and memory. Frontiers in Mollecular Neuroscience. 3, 1 (2010).
- Bronfman, F. C., Lazo, O. M., Flores, C., Escudero, C. A., Lewin, G., Carter, B. Spatiotemporal intracelular dynamics of neurotrophin and its receptors. Implications for neurotrophin signaling and neuronal function. Neurotrophic Factor. Handbook of Experimental Pharmacology. 220, (2014).
- Ascano, M., Bodmer, D., Kuruvilla, R. Endocytic trafficking of neurotrophins in neural development. Trends in Cell Biology. 22 (5), 266-273 (2012).
- Deinhardt, K., Salinas, S., Verastegui, C., Watson, R., Worth, D., Hanrahan, S., Bucci, C., Schiavo, G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 52 (2), 293 (2006).
- Escudero, C. A., et al. c-Jun N-terminal kinase (JNK)-dependent internalization and Rab5-dependent endocytic sorting medaited long-distance retrograde neuronal death induced by axonal BDNF-p75 signaling. Scientific Reports. 9, 6070 (2019).
- Vrabec, J. P., Levin, L. A. The neurobiology of cell death in glaucoma. Eye. 21, 11-14 (2007).
- Liot, G., Zala, D., Pla, P., Mottet, G., Piel, M., Saudou, F. Mutant huntingtin alters retrograde transport of TrkB receptors in striatal dendrites. Journal of Neuroscience. 33 (15), 6298-6309 (2013).
- Zhou, B., Cai, Q., Xie, Y., Sheng, Z. H. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Reports. 2 (1), 42-51 (2012).
- Haubensak, W., Narz, F., Heumann, R., Lessmann, V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. Journal of Cell Science. 111 (11), 1483-1493 (1998).
- Adachi, N., et al. Glucocorticoid affects dendritic transport of BDNF-containing vesicles. Scientific Reports. 5, 12684 (2015).
- Biocompare: The Buyer’s Guide for Life Scientists. Mirus Bio. Cellular Toxicity Caused by Transfection: Why is it important Available from: https://www.biocompare.com/Bench-Tips/121111-Cellular-Toxicity-Caused-by-Transfection-Why-is-it-important/ (2012)
- Zhao, L., et al. Mechanism underlying activity-dependent insertion of TrkB into the neuronal surface. Journal of Cell Science. 122 (17), 3123-3136 (2009).
- Zhao, X., Zhou, Y., Weissmiller, A., Pearn, M., Mobley, W., Wu, C. Real-time imaging of axonal transport of quantum dot-labeled BDNF in primary neurons. Journal of Visualized Experiments. 91, 51899 (2014).
- Sung, K., Maloney, M., Yang, J., Wu, C. A novel method for producing mono-biotinylated, biologically active neurotrophic factors: an essential reagent for single molecule study of axonal transport. Journal of Neuroscience Methods. 200 (2), 121-128 (2011).
- Deerinck, T. The application of fluorescent quantum dots to confocal, multiphoton and electron microscopic imaging. Toxicologic Pathology. 36 (1), 112-116 (2008).
- Unsain, N., Nuñez, N., Anastasia, A., Mascó, D. H. Status epilepticus induces a TrkB to p75 neurotrophin receptor switch and increases brain-derived neurotrophic factor interaction with p75 neurotrophon receptor: an initial event in neuronal injury induction. Neuroscienze. 154 (3), 978-993 (2008).
- Walker, J. M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 32, 5-8 (1994).
- Moya-Alvarado, G., Gonzalez, A., Stuardo, N., Bronfman, F. C. Brain-derived neurotrophic factor (BDNF) regulates Rab5-positive early endosomes in hippocampal neurons to induce dendritic branching. Frontiers in Cellular Neuroscience. 12, 493 (2018).
- Sasi, M., Vignoli, B., Canossa, M., Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers Archiv European Journal of Physiology. 469 (5), 593-610 (2017).
- . The Rab5-Rab11 endosomal pathway is required for BDNF-induced CREB transcriptional regulation in neurons Available from: https://www.biorxiv.org/content/10.1101/844720v1 (2019)
- Mowla, , et al. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. Journal of Biological Chemistry. 276 (16), 12660-12666 (2001).
- Longo, P., Kavran, J., Kim, M. S., Leahy, D. Transient Mammalian Cell Transfection with Polyethyleneimine (PEI). Methods in Enzymology. 529, 227-240 (2013).
- Raymond, C., Tom, R., Perret, S., Moussouami, P., L’Abbé, D., St-Laurent, G., Durocher, Y. A simplified polyethyleneimine-mediated transfection process for large-scale and high-throughput applications. Methods. 55 (1), 44-51 (2011).
- Dalton, A., Barton, W. Over-expression of secreted proteins from mammalian cell lines. Protein Science. 23 (5), 517-525 (2014).
- Hunter, M., Yuan, P., Vavilala, D., Fox, M. Optimization of protein expression in mammalian cells. Current Protocols in Protein Science. 95 (1), 77 (2019).
- Stepanenko, A. A., Heng, H. H. Transient and stable vector transfection: Pitfalls, off-target effects, artifacts. Mutation Research. 773, 91-103 (2017).
- Guerzoni, L. P., Nicolas, V., Angelova, A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharmaceutical Research. 34 (2), 492-505 (2017).
- Angelova, A., Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regeneration Research. 12 (6), 886-889 (2017).

