A Two-Step Pyrolysis-Gas Chromatography Method with Mass Spectrometric Detection for Identification of Tattoo Ink Ingredients and Counterfeit Products
Summary
This method for two-step pyrolysis online coupled to gas chromatography with mass spectrometric detection and data evaluation protocol can be used for multi-component analysis of tattoo inks and discrimination of counterfeit products.
Abstract
Tattoo inks are complex mixtures of ingredients. Each of them possesses different chemical properties which have to be addressed upon chemical analysis. In this method for two-step pyrolysis online coupled to gas chromatography mass spectrometry (py-GC-MS) volatile compounds are analyzed during a first desorption run. In the second run, the same dried sample is pyrolyzed for analysis of non-volatile compounds such as pigments and polymers. These can be identified by their specific decomposition patterns. Additionally, this method can be used to differentiate original from counterfeit inks. Easy screening methods for data evaluation using the average mass spectra and self-made pyrolysis libraries are applied to speed up substance identification. Using specialized evaluation software for pyrolysis GS-MS data, a fast and reliable comparison of the full chromatogram can be achieved. Since GC-MS is used as separation technique, the method is limited to volatile substances upon desorption and after pyrolysis of the sample. The method can be applied for quick substance screening in market control surveys since it requires no sample preparation steps.
Introduction
Tattoo inks are complex mixtures consisting of pigments, solvents, binders, surfactants, thickening agents, and, sometimes, preservatives1. The increased popularity of tattooing in the last decades has led to the establishment of legislation addressing tattoo ink safety across Europe. In most instances, color-giving pigments and their impurities are restricted and therefore should be monitored by state laboratory market surveys to control their compliance with law.
Using the approach of online pyrolysis-gas chromatography mass spectrometry (py-GC-MS) described here, multiple ingredients can be identified simultaneously. Since volatile, semi-volatile and non-volatile compounds can be separated and analyzed within the same process, the variety of target compounds is high compared to other methods used for tattoo ink analysis. Liquid chromatography methods are mostly carried out with pigments solubilized in organic solvents2. Raman spectroscopy as well as Fourier-transform infrared (FT-IR) spectroscopy have been described as suitable tools for the identification of pigments and polymers but are limited with multi-ingredient mixtures since no separation technique is used in standard laboratory applications3,4. Laser desorption/ionization time-of-flight mass spectrometry (LDI-ToF-MS) has also been used for pigment and polymer identification5,6. Altogether, most methods lack the analysis of volatile compounds. The lack of suitable commercial spectral libraries is a common disadvantage of all of these methods. The identification of inorganic pigments has often been carried out with either inductively coupled plasma mass spectrometry (ICP-MS)7,8 or energy dispersive X-ray spectroscopy (EDX)4,9. Also, FT-IR and Raman spectroscopy have been used for the analysis of inorganic pigments such as titanium dioxide or iron oxides in other research fields10,11,12,13.
The goal of this study was to establish a method applicable in standard analytical laboratories with moderate financial costs to upgrade existing and common devices. Py-GC-MS as described here is a non-quantitative approach for identification of organic ingredients from mixtures. Upon identification of suspicious substances in a py-GC-MS screening, target substances can be quantified with more specialized approaches. It is especially interesting for the analysis of non-volatile and non-soluble substances like pigments and polymers.
The described method can be adapted for inks and varnishes in other fields of application. The data evaluation methods described are applicable to all pyrolysis investigations. Also, counterfeit products, mostly from Asian markets, display a potential source of risk to the consumer and a financial burden to manufacturers (personal communication at the 3rd ECTP in Regensburg, Germany, 2017). The method described here can be used to compare the characteristics of putative counterfeit inks to an original bottle, similar to published forensic approaches for car varnish identification14.
Protocol
1. Tattoo ink preparation and sample mounting
- Use a 25 mm hollow glass pyrolysis tube as a sample holder and quartz wool for sample preparation.
- Grab the pyrolysis tube with the specialized tweezers for pyrolysis tubes (bake out for decontamination) and insert the necessary amount of quartz wool with pointed tweezers into the tube.
- Insert two steel sticks (bake out for decontamination) at each side of the pyrolysis tube and compress the wool into a 1–2 mm thick stopper. The stopper must be positioned at the lower third of the pyrolysis tube to achieve adequate heating during the pyrolysis process.
- Ignite a gas burner and bake the pyrolysis tube and filling for 2–3 s from each side to remove contaminants.
NOTE: Use clean gloves and do not touch any item before handling the pyrolysis tube and wool. Use eye protection and remove all flammable liquids and items during the pyrolysis tube bake out. The protocol can be stopped here. Store the prepared pyrolysis tubes in a clean glass dish until further use.
- Shake the tattoo ink bottles vigorously for 1 min by hand to ensure homogeneity. Additionally, they can be placed in an ultrasonic water bath for 1 min. Some inks may still show sedimented pigments after conducting both steps and may need prolonged homogenization.
- Obtain a 2 µL microcapillary with a diameter below the inner diameter of the pyrolysis tube. Dip the capillary tip in the ink and aspirate about 1 µL of ink by filling half of the capillary.
- Insert the capillary into the pyrolysis tube and stain the quartz wool stopper with the ink. A clear color staining must be visible without adding too much ink to the sample.
- Obtain a steel transport adapter for the automated injection unit and attach the prepared pyrolysis tube to it using tweezers for pyrolysis tubes.
- Check that the pyrolysis tube is perfectly vertical and does not fall off during shaking.
- Place the transport adapter in the tray at the desired position.
NOTE: The ink might contaminate the steel transport adapter to which the pyrolysis tube is attached; therefore, this needs more thorough cleaning afterwards.
2. Analysis of ink samples by py-GC-MS
- Set up the GC-MS-system equipped with a Thermal Desorption Unit (TDU) and a pyrolyzer module on top of the Cold Injection System (CIS) according to the manufacturer’s instructions. Use an inert electron impact (EI) ion source at 70 eV and helium with a purity of 99.999% as carrier gas (1 mL/min). Set the split ratio of the CIS to 1:50.
- Install an HP-5MS column and a guard column for separation. Set the following analysis parameters in the instruments’ control software: start the oven temperature at 50 °C, hold for 2 min, and ramp at 10 °C/min to 320 °C. Hold the final temperature for additional 5 min. Set the transfer line temperatures to 320 °C.
- Run each sample in a solvent vent mode without pyrolysis to analyze for semi-volatile compounds.
- Use the solvent vent option of the TDU/injector and ramp the TDU temperature after 0.5 min starting at 50 °C to 90 °C at a rate of 100 °C/min. The solvent vent will be shut off after 1.9 min.
- Heat the temperature of the TDU to 320 °C at a rate of 720 °C/min for 3.5 min. The volatile compounds are captured in the CIS at -150 °C.
- Hold the CIS temperature for 2 min and ramp to 320 °C at a 12 °C/min rate.
NOTE: Extended time between sample preparation and analysis leads to evaporation of volatile compounds since the sample holder is open. Analyze samples within a few hours after preparation if volatile compounds are being targeted.
- Conduct a second run of the same sample in which the pyrolysis unit is used to investigate non-volatile compounds.
- Use the same oven program as for the first desorption run.
- Keep the temperature of the CIS constant at 320 °C and use a split ratio of 1:100.
- Ramp the TDU directly from 50 °C to 320 °C at a 720 °C/min rate and keep constant for 1.6 min.
- Program a 6 s pyrolysis at the desired temperature (here 800 °C) in the final temperature segment of the TDU.
NOTE: Make sure to use an adequate amount of sample and split ratio to prevent contamination of the column and MS. Adapt the split ratios for individual instrument set-ups if necessary.
- Use a polystyrene standard to check the performance of the instrument. Run at least three polystyrene samples before and after each experiment. Insert 2 µL of a 4 mg/mL polystyrene standard in dichloromethane into the glass wool and perform a pyrolysis at 500 °C for 0.33 min.
- Check if the peak area ratio of the polystyrene monomer and the dimer is between 3 and 4 and the tailing of the dimer is below 2 in the resulting chromatogram (also called a pyrogram). Keep historical data of polystyrene parameters and calibrate the pyrolysis temperature if the peak ratio is out of range.
NOTE: Values for polystyrene monomer and dimer ratio as well as tailing should be taken from historical values of well operating systems.
3. Data evaluation approaches
NOTE: Data evaluation should be adapted depending on the individual analytical questions, e.g., the search for volatiles, non-volatile compounds, hazardous cleavage products from azo pigments, or similar.
- Data evaluation for volatile compounds
- For data evaluation of volatile compounds, start the GC-MS analysis/MS library searching software (see Table of Materials) and open the chromatogram of the desorption run.
- Select commercial libraries by clicking on Spectrum and Select Library. Load the library of interest.
- Select integration parameters and perform a library search by clicking on Spectrum and Library Search Report.
NOTE: Take special care to compare library spectra of putative identified compounds with the spectra obtained in the analysis of the ink. Sometimes good matches can be achieved despite the presence of additional molecular mass peaks in the unknown spectra. For unequivocal identification, analytical standards have to be analyzed using the set up and instrument parameters to verify retention times and spectra.
- Fast screening for non-volatile compounds
NOTE: Identification of non-volatile compounds from pyrolysis is based on the presence of multiple specific decomposition products of the parent compound within the same pyrogram. Since pyrograms can contain up to several hundreds of compounds, manual evaluation is hardly feasible. Start with a quick data evaluation approach using the average mass spectra (AMS). This is useful for identification of the most abundant pigment or polymer within the sample. This approach is only suitable for fast screening for ink declaration fraud and for having a starting point for manual pyrogram evaluation.- For pyrolysis data evaluation, mark the whole chromatogram in the GC-MS evaluation software (see Table of Materials) with the right mouse button pressed down to obtain an AMS.
- Create a library of obtained spectra of known reference substances of all compounds of interest, e.g., pigments and polymers, by clicking on Spectrum and Edit Library (click Create New Library if none is present).
- Select Add New Entry and fill in all information of interest.
NOTE: Select the AMS spectra of standards or inks, and click on Spectrum and NIST Search if forwarding to another software capable of MS library searches is desired. - Generate an AMS of the investigated ink pyrogram and use the library search for comparison to the self-made AMS library15. Exclude masses from column bleed or other column noises.
NOTE: The highest match in the library search list will likely be the most abundant pigment or polymer in the inks. To verify this, compare the single characteristic decomposition products of the substance seen in the pyrolysis of the standard substance in the pyrogram of the analyzed ink (see section 3.4 and Supplementary Table 1).
- Identification of non-volatile compounds with specialized pyrogram evaluation software
- Convert the pyrograms of interest to the needed format as stated in the software instructions. Integrate the pyrogram in a way that a maximum of 200 compounds are found. Otherwise, data evaluation time in the pyrogram evaluation sofware increases significantly.
- Build a folder on your computer with all pyrogram entries that should serve as a library, e.g., pigment pyrogram library for pigment identification or pyrograms of an original ink to compare it to putative counterfeit products.
- Load the unknown pyrogram in the tab Library Search by clicking on Esplora.
- Load the library folder and select only MS matching and RT matching in the Search options, since the overall abundance will vary compared to pyrogram of reference pigments.
- Click on advanced in the Search options. Select the parameter “use only peaks with specified MS spectra” and use a fit threshold of 850 in the pyrogram evaluation software.
- Click on Add to save specified MS spectra (3–5 MS spectra of the most abundant peaks) from each pyrogram of reference pigments or polymers from the library in the advanced search options (cf. Supplementary Table 1). In this way only pigment related peaks are compared even in a multi-component ink with otherwise interfering high-abundant peaks.
- Press OK to return to the main window.
- Click on Search to start the comparison.
- If needed, go to Chromatogram Match tab, select a compound, and click Send to NIST to forward the spectra to the MS library software and identify the compound.
NOTE: Click send to MS options to include the spectra in the advanced Search options (cf. step 3.3.6).
- Manual substance identification
NOTE: If no specialized pyrogram evaluation software is available use the data evaluation by standard MS library search program and commercial library together with reported fragments (Supplementary Table 1) and our pyrolysis library15. Manual comparison of pyrogram compounds with known decomposition products is also carried out to verify the hits given in the AMS.- For data evaluation for non-volatile compounds, start the GC-MS analysis/MS library searching software and open the chromatogram of the pyrolysis run.
- Select commercial and (self-made) pyrolysis libraries (e.g., our provided pyrolysis library15) by clicking on Spectrum and Select Library. Load all libraries of interest.
- Integrate the pyrogram in the GC-MS evaluation software and consider all peaks with an area ≥0.2% of the total area (may be adapted in a way that a manageable amount of peaks will be integrated).
- Start the library search by clicking on Spectrum and Library Search Report.
- Manually compare all library matches to specific pigment and polymer decomposition products in Supplementary Table 1 or fragments stated in literature16,17,18,19,20,21,22,23,24,25,26. For pigments, 2 to 3 decomposition products are needed to unambiguously identify the pigment used.
NOTE: All spectral matches with corresponding libraries must be carefully evaluated. Additional peaks higher than the mass peak often account for similar structures with additional side groups. If possible, reference substances should be analyzed to obtain the individual retention time in the analytical set-up.
Representative Results
The method includes a two-step chromatographic approach for each sample (Figure 1). In the first run, the sample is dried inside the injector system at 90 °C before volatile compounds are transferred onto the column. Since the drying process is incomplete in most cases, residual solvents and volatile compounds are transferred and analyzed. In the second run, the previously dried sample is subsequently pyrolyzed to facilitate the analysis of non-volatile organic components.
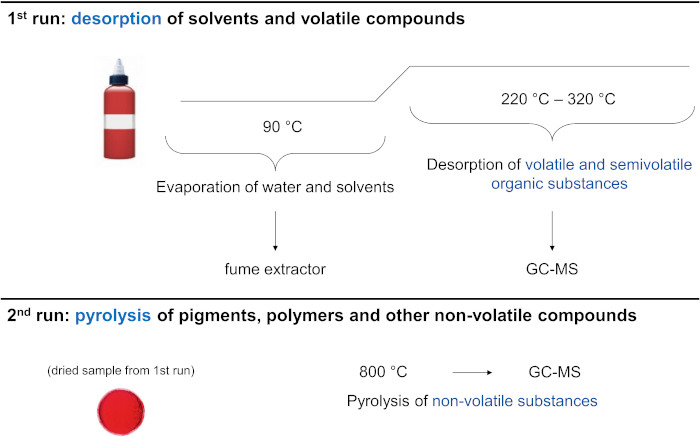
Figure 1: Schematic diagram of the pyrolysis workflow. Please click here to view a larger version of this figure.
Well produced inks with highly pure ingredients and a limited number of components result in chromatograms easy to interpret with standard libraries, since most peaks can be identified (Figure 2). But even in the high-quality inks, non-declared ingredients have been found. For example, propylene glycol is often found in addition to the declared glycerol (Figure 2 and Figure 3).
Other substances such as formaldehyde might be added as a preservative. 1-Hydroxy-2-propanone may result as an impurity of pigment synthesis and is therefore an example of a non-intentionally added substance (NIAS).
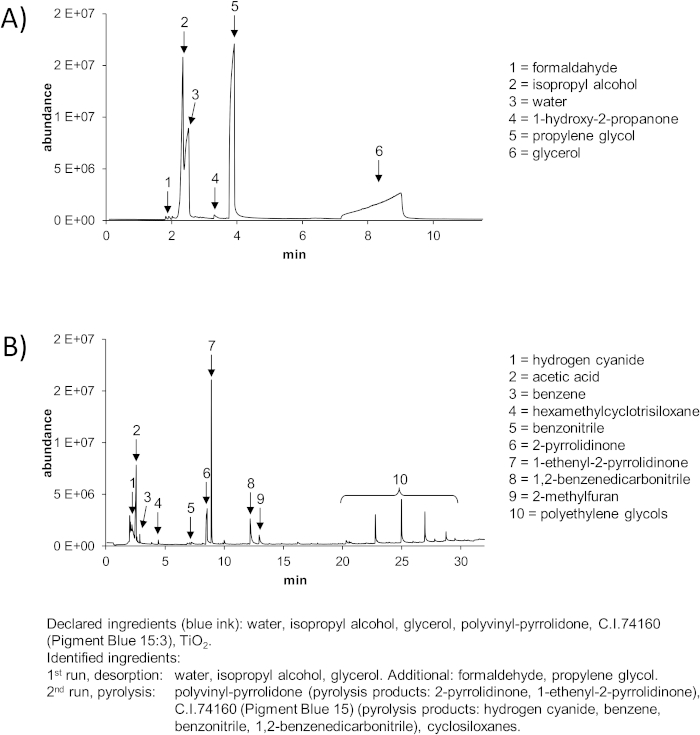
Figure 2: Representative results of the py-GC-MS analysis of a tattoo ink with only a few, pure ingredients. (A) 1st run: desorption for identification of volatile compounds. (B) 2nd run: pyrolysis for identification of less and non-volatile compounds. Declared and identified ingredients are indicated below. Please click here to view a larger version of this figure.
Inks containing multiple ingredients and impurities will result in a pyrogram that is difficult to interpret (Figure 3). Most peaks occurring in the second run may not be baseline separated from each other, making identification difficult, even when utilizing data deconvolution. Some substances might also result in peaks below the threshold set during data evaluation (e.g., 0.2% of total peak area). A solution to this problem might be a step-wise approach using 400 °C, 600 °C, and 800 °C in consecutive pyrolysis steps for the very same sample (see Figure 4).
Some pigment decomposition products may descent from multiple pigments (Supplementary Table 1). For example in Figure 3 and Figure 4, acetyl cyanide can derive from multiple yellow or orange pigments. The degradation product 2-methoxyphenylisocyanate may also derive from the pigment Red 9, and o-anisidine from the pigments Red 170 and Red 9. However, due to the combination with the degradation product 4-methoxy-2-nitro-aniline and the yellow appearance of the ink, only pigments yellow 65 and 74 would be plausible as parent compounds. These two pigments are regional isomers and cannot be distinguished from another with this method. Pigment orange 13 — which was declared on the ink bottle — has not been identified. If the pigment was only present in low amounts, it might have been below the limit of detection. On the other hand, inks are often declared falsely27.
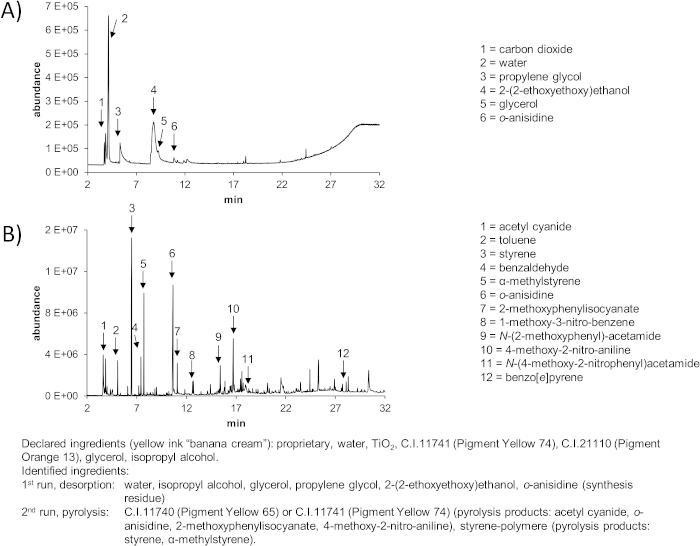
Figure 3: Representative results of the py-GC-MS analysis of the yellow tattoo ink “banana cream” with many, impure ingredients. (A) 1st run: desorption for identification of volatile compounds. (B) 2nd run: pyrolysis for identification of less and non-volatile compounds. Declared and identified ingredients are indicated below. Please click here to view a larger version of this figure.
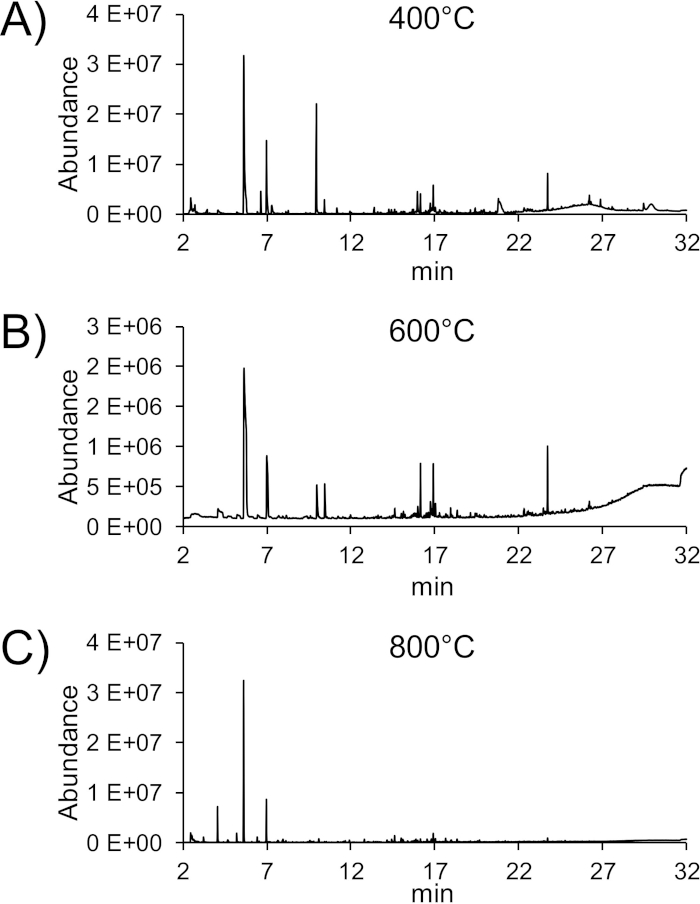
Figure 4: Gradual pyrolysis of the yellow ink “banana cream” displayed in Figure 3. A-C) Consecutive pyrolysis runs at 400 °C, 600 °C, and 800 °C. Please click here to view a larger version of this figure.
A positive result for counterfeit product identification is displayed in the following example (Figure 5). Three “lemon yellow” inks have been purchased either from a licensed distributor of the US-based ink manufacturer, via an internet auction platform or via an Asian market place. All inks have been analyzed with the two-step py-GC-MS method. In this example, the differences in peak numbers and retention times are already visible by eye.
The chromatogram from the 1st desorption run and the pyrogram from the 2nd run of the original ink were compared against three independent acquisitions of the original ink and the two counterfeit products using pyrogram evaluation software. The software was found to be highly useful in distinguishing the different inks. The forward match factor was above 0.9 (with 1 being the perfect match) only towards pyrograms or desorption chromatograms of the same ink, respectively.
Also, forward matches above 0.9 were only achieved with the same ink when comparing the ink to library contained pyrograms of 84 inks of various colors and manufacturers.
Alternatively, a statistical comparison as proposed by Yang et al. for car varnishes may be applied14.
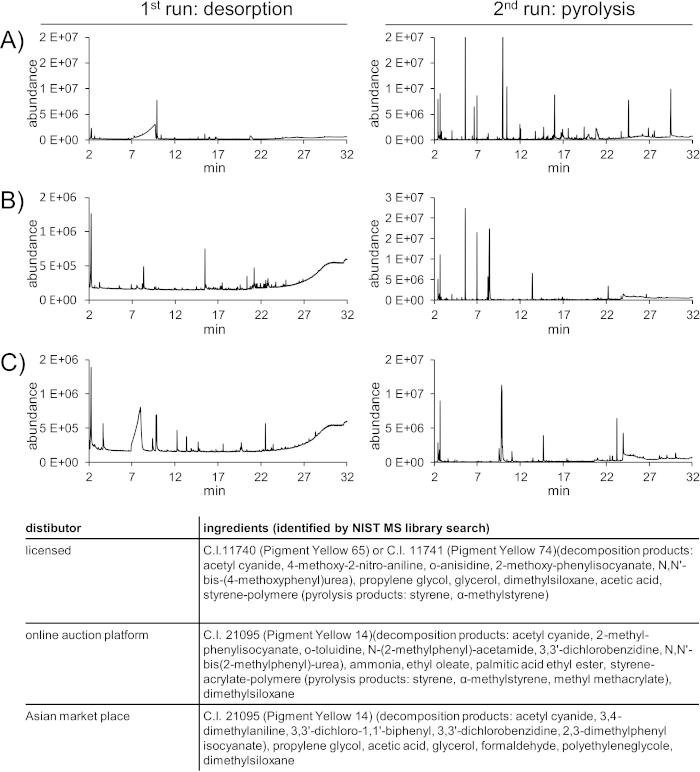
Figure 5: Identification of counterfeit products by py-GC-MS. Three “lemon yellow” inks from licensed distributor (A), an online auction platform (B), and an Asian market place (C) have been analyzed. Please click here to view a larger version of this figure.
Discussion
Py-GC-MS is a useful screening method for a broad range of substances in tattoo inks that can also be used for the analysis of other products. Compared to other methods, py-GC-MS can be conducted with only minimal sample preparation. GC-MS devices can be found in most analytical laboratories compared to more specialized methods such as MALDI-ToF-MS and EDX.
The data evaluation of pyrograms may be challenging, since the list of possible ingredients is infinite in theory and library searches that also account for the combination of substances towards a parent compound in the library are necessary. The data evaluation methods described here allow for reliable fast screening of substances that have been added to standard pyrogram libraries. Conversely, testing for counterfeit products is a fast and straightforward approach that can be conducted without any pre-build libraries, since the identification of single substances is irrelevant.
To obtain the best possible results, the amount of ink added to pyrolysis should neither be too high nor too low. This will either result in a contamination of the pyrolysis unit, liner or column or a lack of significant peaks for proper pyrogram interpretation. Therefore, using a defined volume of ink as described in this method with adjusted split ratios is highly recommended. As shown in Figure 3, impurities or polymers can overload the pyrogram with peaks impairing the identification of single substances. Therefore, the detection limit for pigments is highly dependent on the corresponding mixtures. In such cases, pigments might first be separated from other ink ingredients by dilution and precipitation in alcohol-water solvents.
The limitation of the method is the analysis of organic pigments without specific cleavage sides such as quinacridones, perylenes and perinones16,17,18. Also, if a mixture of multiple pigments with the same cleavage group occurs (e.g., with azo pigments), the identification might be challenging (cf. ink displayed in Figure 3). In addition, the pyrolysis products must be able to enter the gas phase. Polymers like hydroxyethyl-cellulose consisting of sugar monomers that have to be chemically modified for GC-MS analysis cannot be detected by py-GC-MS. As in all other methods, only pigments with known pyrograms can be identified. However, main decomposition products can be concluded from the pigment structure, especially in the case of azo pigments. Therefore, checking for plausibility of a declared pigment can be carried out even if the pigment has never been analyzed in py-GC-MS before.
The method can be used to discriminate original inks from counterfeit products. However, a reliable sample of the original ink must be available. Since the composition of inks may change over time, inks produced in the same time range or at best from the same batch must be used for comparison. In future, py-GC-MS might be used to monitor tattoo ink ingredients and thereby reveal declaration fraud and the use of banned pigments and possible other ingredients. A further application of these methods might be the identification of counterfeit products14.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the intramural research project (SFP #1323-103) at the German Federal Institute for Risk Assessment (BfR).
Materials
| 99.999% Helium carrier gas | Air Liquide, Düsseldorf, Germany | – | |
| 5975C inert XL MSD with Triple-Axis Detectors | Agilent Technologies, Waldbronn, Germany | – | |
| 7890A gas chromatograph | Agilent Technologies, Waldbronn, Germany | – | |
| AMDIS software (Version 2.7) | The National Institute of Standards and Technology, Gaithersburg, MD, USA | – | can be used for GC/MS peak integration, e.g. for transfer to pyrogram evaluation software |
| Cold Injection System (CIS) | Gerstel, Mühlheim, Germany | – | |
| electron impact (EI) source | Agilent Technologies, Waldbronn, Germany | – | |
| Enhanced ChemStation (E02.02.1431) | Agilent Technologies, Waldbronn, Germany | – | used to generate Average Mass Spektra (AMS), can be used for peak integration and standard GC/MS library search |
| J&W HP-5MS GC Column, 30 m, 0.25 mm, 0.25 µm, 5975T Column Toroid Assembly | Agilent Technologies, Waldbronn, Germany | 29091S-433LTM | |
| MassHunter Software | Agilent Technologies, Waldbronn, Germany | – | no Version specified, can be used for GC/MS peak integration and standard GC/MS library search |
| Microcapillary tube Drummond Microcaps, volume 2 µL | Sigma-Aldrich, St. Louis, MO, USA | P1549-1PAK | |
| MS ChromSearch (Version 4.0.0.11) | Axel Semrau GmbH & Co. KG, Sprockhövel, Germany | – | specialized pyrogram evaluation software |
| NIST MS Search Program (MS Search version 2.0g) | The National Institute of Standards and Technology, Gaithersburg, MD, USA | – | used for MS and AMS library generation and corresponding substance search with selfmade and commercial libraries |
| NIST/EPA/NIH Mass Spectral Library (EI) mainlib & replib (Data version: NIST v11) | The National Institute of Standards and Technology, Gaithersburg, MD, USA | – | used commercial mass spectral library |
| Polystyrene (average Mw ~192,000) | Sigma-Aldrich, St. Louis, MO, USA | 430102-1KG | |
| Pyrolysis tubes, tube type – quartz glass – lenght 25 mm; 100 Units | Gerstel, Mühlheim, Germany | 018131-100-00 | |
| Pyrolyzer Module for TDU | Gerstel, Mühlheim, Germany | – | |
| Quartz wool | Gerstel, Mühlheim, Germany | 009970-076-00 | |
| Steel sticks | Gerstel, Mühlheim, Germany | – | |
| Thermal Desorption Unit (TDU 2) | Gerstel, Mühlheim, Germany | – | |
| Transport adapter | Gerstel, Mühlheim, Germany | 018276-010-00 | |
| Tweezers for Pyrolysis tubes | Gerstel, Mühlheim, Germany | 009970-074-00 | |
| Zebron Z-Guard Hi-Temp Guard Column, GC Cap. Column 10 m x 0.25 mm, Ea | Phenomenex Ltd. Deutschland, Aschaffenburg, Germany | 7CG-G000-00-GH0 |
Riferimenti
- Dirks, M., Serup, J., Kluger, N., Bäumler, W. . Tattooed skin and health. Vol. 48. Current Problems in Dermatology. , 118-127 (2015).
- Engel, E., et al. Establishment of an extraction method for the recovery of tattoo pigments from human skin using HPLC diode array detector technology. Analytical Chemistry. 78 (15), 6440-6447 (2006).
- Poon, K. W. C., Dadour, I. R., McKinley, A. J. In situ chemical analysis of modern organic tattooing inks and pigments by micro-Raman spectroscopy. Journal of Raman Spectroscopy. 39 (9), 1227-1237 (2008).
- Timko, A. L., Miller, C. H., Johnson, F. B., Ross, V. In vitro quantitative chemical analysis of tattoo pigments. Archives of Dermatology. 137, 143-147 (2004).
- Boon, J. J., Learner, T. Analytical mass spectrometry of artists’ acrylic emulsion paints by direct temperature resolved mass spectrometry and laser desorption ionisation mass spectrometry. Journal of Analytical and Applied Pyrolysis. 64, 327-344 (2002).
- Hauri, U. Inks for tattoos and permanent make-up / pigments, preservatives, aromatic amines, polyaromatic hydrocarbons and nitrosamines. Department of Health, Kanton Basel-Stadt. Swiss National Investigation Campaign. , (2014).
- Bocca, B., Sabbioni, E., Mičetić, I., Alimonti, A., Petrucci, F. Size and metal composition characterization of nano- and microparticles in tattoo inks by a combination of analytical techniques. Journal of Analytical Atomic Spectrometry. 32 (3), 616-628 (2017).
- Schreiver, I., et al. Synchrotron-based nano-XRF mapping and micro-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Scientific Reports. 7, 11395 (2017).
- Taylor, C. R., Anderson, R. R., Gange, R. W., Michaud, N. A., Flotte, T. J. Light and electron microscopic analysis of tattoos treated by Q-switched ruby laser. Journal of Investigative Dermatology. 97, 131-136 (1991).
- Namduri, H., Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corrosion Science. 50 (9), 2493-2497 (2008).
- Burgio, L., Clark, R. J., Hark, R. R. Raman microscopy and x-ray fluorescence analysis of pigments on medieval and Renaissance Italian manuscript cuttings. Proceedings of the National Academy of Sciences of the United States of America. 107 (13), 5726-5731 (2010).
- Manso, M., et al. Assessment of toxic metals and hazardous substances in tattoo inks using Sy-XRF, AAS and Raman spectroscopy. Biological Trace Element Research. 187 (2), 596-601 (2017).
- Yakes, B. J., Michael, T. J., Perez-Gonzalez, M., Harp, B. P. Investigation of tattoo pigments by Raman spectroscopy. Journal of Raman Spectroscopy. 48 (5), 736-743 (2017).
- Yang, S. -. H., Shen, J. Y., Chang, M. S., Wu, G. J. Differentiation of vehicle top coating paints using pyrolysis-gas chromatography/mass spectrometry and multivariate chemometrics with statistical comparisons. Analytical Methods. 7, 1527-1534 (2015).
- Schreiver, I., Hutzler, C., Luch, A. Data from: Two-step pyrolysis-gas chromatography method with mass spectrometric detection for identification of tattoo ink ingredients and counterfeit products. Dryad Digital Repository. , (2019).
- Schreiver, I., Hutzler, C., Andree, S., Laux, P., Luch, A. Identification and hazard prediction of tattoo pigments by means of pyrolysis—gas chromatography/mass spectrometry. Archives of Toxicology. 90 (7), 1639-1650 (2016).
- Ghelardi, E., et al. Py-GC/MS applied to the analysis of synthetic organic pigments: characterization and identification in paint samples. Analytical and Bioanalytical Chemistry. 407 (5), 1415-1431 (2015).
- Russell, J., Singer, B. W., Perry, J. J., Bacon, A. The identification of synthetic organic pigments in modern paints and modern paintings using pyrolysis-gas chromatography-mass spectrometry. Analytical and Bioanalytical Chemistry. 400 (5), 1473-1491 (2011).
- Silva, M. F., Domenech-Carbo, M. T., Fuster-Lopez, L., Mecklenburg, M. F., Martin-Rey, S. Identification of additives in poly(vinylacetate) artist’s paints using PY-GC-MS. Analytical and Bioanalytical Chemistry. 397 (1), 357-367 (2010).
- Peris-Vincente, J., Baumer, U., Stege, H., Lutzenberger, K., Gimeno Adelantado, J. V. Characterization of commercial synthetic resins by pyrolysis-gas chromatography/mass spectrometry: application to modern art and conservation. Analytical Chemistry. 81, 3180-3187 (2009).
- Kleinert, J. C., Weschler, C. J. Pyrolysis gas chromatographic-mass spectrometric identification of polydimethylsiloxanes. Analytical Chemistry. 52 (8), 1245-1248 (1980).
- Scalarone, D., Chiantore, O. Separation techniques for the analysis of artists’ acrylic emulsion paints. Journal of Separation Science. 27 (4), 263-274 (2004).
- Sonoda, N. Characterization of organic azo-pigments by pyrolysis-gas chromatography. Studies in Conservation. 44, 195-208 (1999).
- Chiantore, O., Scalarone, D., Learner, T. Characterization of artists’ crylic emulsion paints. International Journal of Polymer Analysis and Characterization. 8 (1), 67-82 (2003).
- Schossler, P., Fortes, I., Figueiredo Júnior, J. C. D., Carazza, F., Souza, L. A. C. Acrylic and Vinyl Resins Identification by Pyrolysis-Gas Chromatography/Mass Spectrometry: A Study of Cases in Modern Art Conservation. Analytical Letters. 46 (12), 1869-1884 (2013).
- Wallisch, K. L. Pyrolysis of random and block copolymers of ethyl acrylate and methyl methacrylate. Journal of Applied Polymer Science. 18, 203-222 (1974).
- Hauri, U. Inks for tattoos and PMU (permanent make-up) / Organic pigments, preservatives and impurities such as primary aromatic amines and nitrosamines. State Laboratory of the Canton Basel City. , (2011).

