Preparation of Mouse Retinal Cryo-sections for Immunohistochemistry
Summary
This report describes comprehensive methods for preparing frozen mouse retina sections for immunohistochemistry (IHC). Methods described include dissection of the ocular posterior cup, paraformaldehyde fixation, embedding in Optimal Cutting Temperature (OCT) media and tissue orientation, sectioning and immunostaining.
Abstract
Preparation of high-quality mouse eye sections for immunohistochemistry (IHC) is critical for assessing the retinal structure and function and for determining the mechanisms underlying retinal diseases. Maintaining structural integrity throughout the tissue preparation is vital for obtaining reproducible retinal IHC data but can be challenging due to the fragility and complexity of retinal cytoarchitecture. Strong fixatives like 10% formalin or Bouin’s solution optimally preserve the retinal structure, they often impede IHC analysis by enhancing the background fluorescence and/or diminishing antibody-epitope interactions, a process known as epitope masking. Milder fixatives, on the other hand, like 4% paraformaldehyde, reduces background fluorescence and epitope-masking, meticulous dissection techniques must be utilized to preserve the retinal structure. In this article, we present a comprehensive method to prepare mouse ocular posterior cups for IHC that is sufficient to preserve most antibody-epitope interactions without loss of retinal structural integrity. We include representative IHC with antibodies to various retinal cell type markers to illustrate tissue preservation and orientation under optimal and sub-optimal conditions. Our goal is to optimize IHC studies of the retina by providing a complete protocol from ocular posterior cup dissection to IHC.
Introduction
Immunohistochemistry (IHC) is a powerful technique for localizing specific proteins and cellular structures in tissues in situ1,2,3. Inappropriate fixation methods and sub-optimal sectioning of complex tissues can disrupt tissue structure, generate high background staining or diminish antibody-epitope interactions, resulting in staining artifacts and consequent misinterpretation of IHC data4. As the vertebrate retina is a complex and highly organized neural organ composed of strata of interconnected photoreceptors, interneurons and ganglion cells, it is very fragile and can be easily disrupted during dissection and sectioning. A detailed, standardized, and validated protocol from mouse eye dissection and orientation to immunostaining will help significantly reduce IHC artifacts, thereby, increasing the reliability of the results and allowing for more accurate comparative data analysis.
There are many protocols for tissue preparation for IHC, however, not all are suitable for retinal tissue. Strong fixatives like 10% formalin or Bouin's solution preserve retinal structure during dissection and sectioning5. Unfortunately, strong fixatives often lead to the enhanced background fluorescence and epitope masking due to the chemical modification of epitopes6. On the other hand, milder fixatives, like 4% paraformaldehyde (PFA) can alleviate some of these artifacts but require meticulous dissection and sectioning to preserve the optimal retinal structure. PFA rapidly penetrates tissue, but cross-links proteins very slowly, reducing the risk of epitope masking. Since short time PFA incubation is a relatively mild fixation, tissues often require rapid freezing to preserve antigens. It is important to avoid ice crystal formation during tissue freezing as they distort and damage the integrity of cells and tissues7.
Here we describe detailed and standardized protocols for dissection, fixation, and cryo-protection of mouse ocular posterior cups that yield consistent and reliable IHC data.
Protocol
All methods described here were carried out in strict accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals provided by the Institute of Laboratory Animal Resources and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
All tools and equipment for the methods are shown in Figure 1 and listed in the Table of Materials.
1 . Mouse eye enucleation, eye cup dissection, and embedding
- Euthanize mice with carbon dioxide (CO2) followed by either decapitation (postnatal mice P0 – P11) or cervical dislocation (adult >P11 mice). For P0-P14 mice, eyes are covered by skin. Ensure to remove the skin prior to subsequent steps.
- Mark the temporal part of the eye (Figure 2A) by slightly burning the cornea using a tail cauterizer. Avoid burning a hole in the cornea by touching the cornea very lightly and for no more than a split second with the cauterizer.
- Immediately enucleate mouse eyes using curved Dumont #5/45 forceps. Incubate for 15 min in a 1.5 mL cryotube tube with 1 mL of 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) on ice.
- After 15 min in 4% PFA, transfer the fixed eye using the curved forceps Dumont #5/45 to a modified 35 mm dissection dish (see Table of Materials and Figure 1C) filled with PBS and place under dissecting microscope.
- Make a small incision at the burn mark, perpendicular to and just above the limbus (border of the cornea) using the micro knife.
- Insert the curved scissors into the incision and perform a circumferential cut of the cornea following the limbus (Figure 2B).
- Remove the cornea and extract the lens with a thin Dumont #5 forceps, (Figure 2B) and lift it delicately away from the posterior portion of the eye. The vitreous body, the clear gel that fills the space between the lens and the retina, will come out with the lens (Figure 2B) and the retina will be visible as a white surface covering the inside of the posterior eye cup. Ensure that there is no visible damage to the retina, such as holes or tears (Figure 2C).
- Wash the eye cups twice in 1 mL of PBS for 10 min at room temperature.
- Cryoprotect the eye cups by equilibrating them in a solution of 15% sucrose in PBS overnight at 4 °C until the eye cup sinks
- Transfer the eye cups to 30% sucrose in PBS for 2-3 h until the eye cup sinks.
- Embed the eye cups in OCT in 10 x 10 mm cryomolds (Figure 3A). Using a dissecting microscope, orient the eye in the cryomold along its dorsal-ventral axis (Figure 2D, Figure 3A) by rotating the eye until the burn mark is on top, the optic nerve is on the left and the cut-out part of the mold faces the right (Figure 2D). Take care to avoid bubbles near the tissue.
NOTE: To manipulate the eye cup, use a titanium probe made of a titanium wire attached to a pipette tip. - To snap-freeze retinal tissue, immerse the cryomold for at least 5 min in a metal beaker containing isopentane and place the beaker in liquid nitrogen or dry ice/100% ethanol bath (up to 1/3 of the height of the metal beaker).
NOTE: The cryomold should be immersed in isopentane to greater than half of its height but does not have to be completely submerged. - Remove the frozen block and wrap it in aluminum foil.
- Store at -80 °C.
- Mark the frozen block using a pen or sharpie to record the orientation of the block (Figure 3B).
2 . Sectioning using a cryostat
- After adjusting the cryostat temperature (between -20 and -25 °C), allow the molds containing the embedded eye cups to equilibrate to the cryostat temperature for 1 h.
- Install the block with dorso-ventral orientation (Figure 3C) and cut 10 µm thick serial sections. Carefully place the retinal sections on annotated and numbered microscope slides.
- Store slides in slide boxes at -20 °C or -80 °C until IHC procedures.
3 . Fluorescence immunostaining using the Slide Rack
NOTE: The Slide Rack system (e.g., Sequenza) holds the glass microscope slides with a cover plate, creating a ~100 µL capillary gap between the slide and the plate.
- Thaw the cryo-sections at room temperature (RT) for 2 h to 4 h to allow them to dry and attach to microscope slides.
NOTE: It is also possible to dry them at 30-37 °C for 30 min to 1 h. - Place slides in Slide Rack and wash the retinal sections twice in 100-200 µL PBS for 2 min.
- Permeabilize the retinal sections by incubating them in 0.25% Triton-X / 0.05% NaN3in PBS for 5 min at RT.
- Add 100 µL of blocking solution to the slides.
- Add 100 µL of primary antibody diluted in blocking solution to the slides.
- Incubate retinal sections overnight at 4 ˚C.
NOTE: During this time, cover the Slide Rack to avoid desiccation of the sections. - Wash the retinal sections 3 times, each for 15 min, using 200 µL PBS.
NOTE: The addition of 0.001- 0.002% Triton-X100 to PBS (PBS-T) allow a more stringent wash but can disrupt some membranes and cellular structures. - Incubate retinal sections in fluorescent-labeled secondary antibodies for 1 h at RT. From now on protect from light.
- Wash the retinal sections 3 times, each for 15 min.
- Incubate the retinal sections at RT for 5 min with a nuclear marker such as Hoechst 33342 or DAPI solution.
- Wash the retinal sections 1 time for 15 min.
- Place a coverslip on top of a paper towel on the lab bench.
- Add 2 drops of anti-fading mounting media to the center of the coverslip.
- Turn the slide over to have the retinal cryo-section facing down.
- Carefully mount the coverslip slowly, at a ~45˚ angle, tip the slide onto the mounting media.
NOTE: Avoiding forming bubbles as you lower the slide in place. - Apply even pressure, using a heavy book or catalog (see below), to the slide-coverslip combination to eliminate excess mounting media.
NOTE: To ensure consistency in sample preparation, always use the same object, (i.e., the same book or catalog) to apply even pressure to the coverslip during slide mounting. - Keep flat and allow to harden overnight at RT in the dark. Store slides flat at 4 °C indefinitely.
- Image via wide field or confocal fluorescence microscopy (Figure 4).
Representative Results
To illustrate how these protocols, ensure optimal retinal preservation for IHC, we probed retinal sections from P28 WT mice (C57BL/6N) with antibodies to rhodopsin (a photoreceptor marker)8, glutamic acid decarboxylase 65 (Gad65, an amacrine cell marker)9, glutamine synthetase (GS, a Müller cell marker)10, and calbindin (a horizontal cell marker)11 (Figure 4). IHC indicate that our protocols consistently yield high quality and well-preserved retinal sections. Notably, the rhodopsin-rich inner and outer segments remain vertical and intact with little to no separation from overlying the retinal pigmented epithelium (RPE) (Figure 4A). In addition, Müller cells remain well-preserved with soma properly aligned and cytoplasmic processes that span from the ganglion cell layer (GCL) to the outer nuclear layer (ONL) (Figure 4B).
Interneurons, such as Gad65-positive amacrine cells, also remain properly stratified within the INL and inner plexiform layer (IPL) (Figure 4B) while well-defined horizontal cells are detectable at the INL and outer plexiform layer (OPL) border (Figure 4C). Collectively, these data demonstrate that our method preserves retinal cell and tissue integrity, from photoreceptors to ganglion cells
To facilitate transverse sectioning, it is important to meticulously orientate the optical cup in the cryo-mold prior to freezing. Misoriented retinas, poor sectioning technique or sectioning with dull or damaged microtome knives can cause artifacts such as retinal tissue tears, distortions, and cellular displacement, as in Figure 4. Examples of section artifacts are shown in Figure 4D-F. In Figure 4D poor sectioning and alignment lead to photoreceptor inner and outer segments distortion (Figure 4D) and small tissue tears (Figure 4E, arrow). In another example, poor tissue orientation prior to section leads to sub-optimal alignment of Muller cell soma and cytoplasmic processes Figure 4E. Figure 4F shows how poor sectioning likely caused by a dull microtome knife caused horizontal cells to aberrantly smear from the OPL to the GCL. Thus, precise attention must be applied to the execution of each step outlined in the protocol to ensure the highest quality and reproducibility of IHC data.
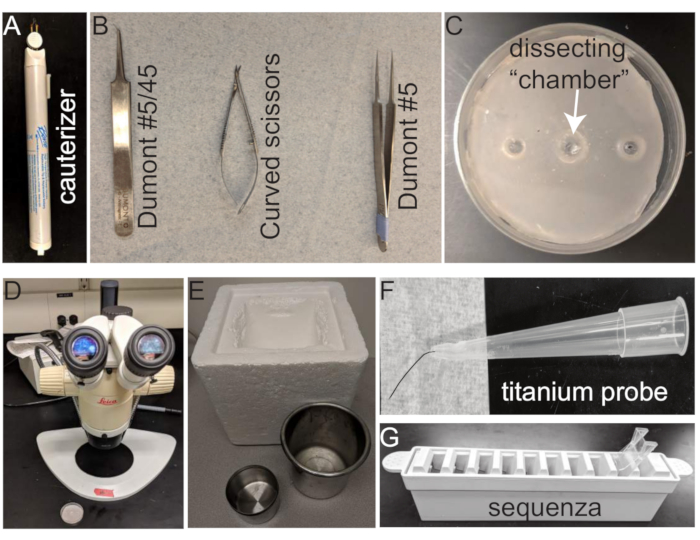
Figure 1: Tools for mouse eye dissection, embedding, and IHC. (A) Cauterizer; (B) Dissection tools from left to right: Dumont #5/45 curved forceps, Curved scissors, and Dumont #5 thin forceps; (C) 35 mm dissection dish (arrow points to dissecting chamber); (D) Dissecting microscope; (E) Frozen tools from left to right: Insulated cooler, stainless steel beaker for isopentane bath , stainless steel liquid N2 bath; (F) titanium probe; (G) Slide Rack and Coverplate for IHC. Please click here to view a larger version of this figure.
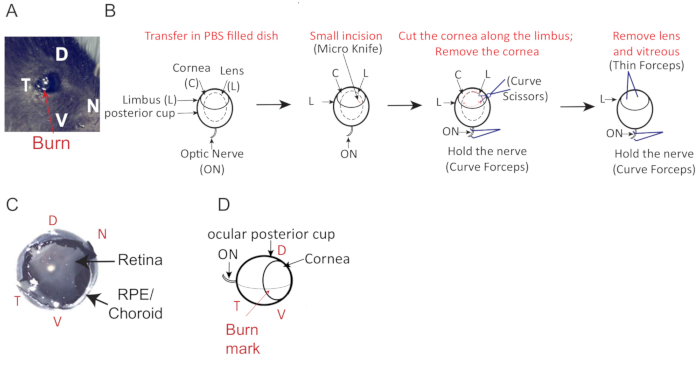
Figure 2: Mouse eye cup orientation and description. (A) Temporal burn mark location (red arrow) to facilitate eye orientation (dorso = D, ventral = V, temporal = T, nasal = N). (B) Schematic for mouse eye dissection. A small incision is made at the burn mark, perpendicular into the cornea and just above the limbus using a fine scalpel (Micro knife). Cut around the cornea to separate the anterior and posterior part of the eye. (C) Eye cup with an intact retina. (D) Dorso-ventral orientation of mouse eye with the optic nerve (ON), lens (L), cornea (C) the limbus (OS). Please click here to view a larger version of this figure.
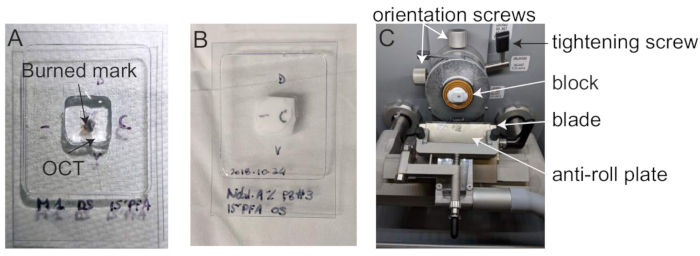
Figure 3: Mouse eye embedding. (A) Dorso-ventral orientated mice eye cup in cryomold filled with OCT, (B) Frozen mouse retinal cup in annotated cryomold. (C) Cryostat. Please click here to view a larger version of this figure.
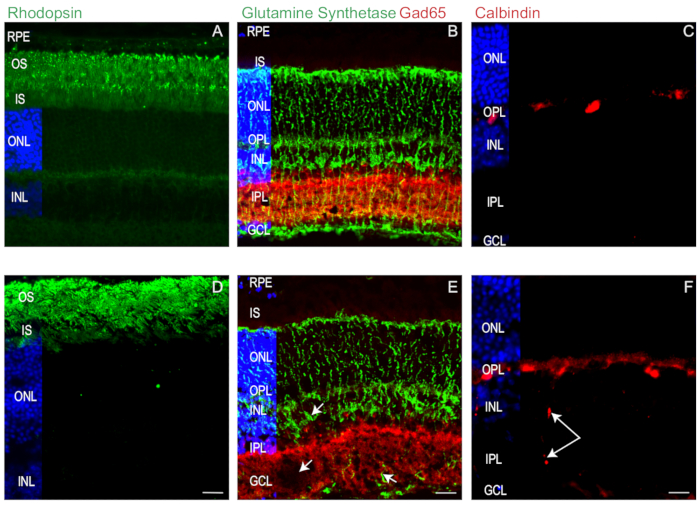
Figure 4: Mouse retina immunohistochemistry.(A-C) Good quality and (D-F) poor quality retinal sections from P28 WT were labeled with antibodies to the rhodopsin (A, C) amacrine marker GAD65 (B, E) and the Müller cell marker glutamine synthetase (GS; B, E), and horizontal cell marker calbindin (C, F). White arrows point to tissue tears and aberrant smearing of the tissue. Scale bar, 20 µm. Nuclei labeled with Hoechst 33342 (blue). Please click here to view a larger version of this figure.
Discussion
Mouse retina dissection is a delicate process due to the small size and shape of mouse eyes and the fragility of retinal tissue. Even though performing high-quality dissection is a matter of practice, having a detailed protocol, providing efficient methods and tips is a necessity to obtain retinal sections and IHC. In addition to the protocols described here, there are several tips that allow for consistent high-quality retinal sections that are suitable for reproducible IHC.
Prior to commencing with dissection, it is important to be comfortable, relaxed and set the room lighting for optimal vision during mouse eye microdissection. To maintain mouse eye cup shape while dissecting the eye, we suggest keeping eyes submerged in PBS in custom made wax-coated dissection dishes. We suggest coating a 35mm dissection dish with light pink colored dental wax (Figure 1C). Indeed, the light pink color of the dental wax provides adequate contrast to see readily eye structures under a dissection scope, the eye-sized impressions in the wax, made by pressing the base of microfuge tubes into the wax, will keep the eye cup in place while allowing the appropriate rotation of the tools around the eye cup during the dissection. In addition, it is important to remove as much vitreous fluid from the eye cup as possible while removing the lens, as residual vitreous fluid is prone to form tissue damaging ice crystals during the tissue block freezing step. We generally use a tissue paper and place it to the border of the dissected eye cup, to carefully remove all remaining vitreous fluid by capillary without disturbing the retina tissue. In the unfortunate case where a small amount of residual vitreous fluid is still present in the eye cup, sectioning should be done at -20 °C to reduce transformation of the vitreous in ice and therefore preserve the sharpness of the blade and the quality of the sectioning.
Fixation and embedding methods also have a major impact on the quality of the sections and the IHC. Although formalin-fixed paraffin-embedded sections yield optimal structural integrity of the eye tissue, formalin contains methanol that can enhance background fluorescence. To avoid these artifacts, we recommend using fresh EM grade paraformaldehyde (PFA) to limit background and autofluorescence caused by methanol or oxidized PFA4,5,6. We recommend the mild fixation conditions described in our protocol fixing the eye with 4% paraformaldehyde for 15 min on ice. Since PFA rapidly penetrates the tissue, but cross-links proteins very slowly, those conditions are sufficient to preserve retinal structural integrity during the dissection and preserve the antigenicity of common retinal antigens without the need for antigen retrieval methods4. If the retina structure is not well preserved, the duration of PFA fixation can be increased, however it is important to be aware that over-fixating tissues will enhance non-specific background fluorescence and can lead to epitope masking, while under-fixating tissues may compromise structural integrity of the tissue and cause the loss of some antigens prone to proteolytic degradation. Since short time PFA incubation is a relatively mild fixation, we also recommend snap-freezing retinas by dipping the OCT-embedded eye, contained in a cryomold (10 mm x 10 mm x 5 mm), in an isopentane bath immersed in liquid nitrogen. Snap-freezing tissues will preserve antigens, while sucrose solution and OCT act as cryo-protectants. This method will reduce the distortion of the tissue due to the formation of ice crystals, which can cause the so-called Swiss Cheese type of tissue damage. The cryomold should not be dipped directly in liquid nitrogen or it will start to boil, forming a vapor barrier and that will slow the freezing process, resulting in a heterogeneous fixation and preservation. Moreover, tissue and OCT in contact with liquid nitrogen can crack, thereby affecting the integrity of the eye7. We, therefore, recommend dipping the cryomold (10 mm x 10 mm x 5 mm) in no more than 5 mm deep isopentane using a small stainless-steel beaker, itself dipped in a larger stainless-steel beaker (100 mm wide minimum) containing liquid nitrogen. To avoid the rapid evaporation of the liquid nitrogen during the preparation of several samples, we keep both baths in an insulated cooler. It should be noted that cryopreserving tissues in OCT is not a long-term preservation method, due to the formation of ice crystals in the tissue over time which can alter subcellular morphology and/or denature some antigens. Consequently, tissue blocks and frozen sections should be stored at -20 °C for short-term storage and for -80 °C long-term storage.
The mammalian retina is an asymmetric organ with a specific spatial distribution of the cells across the temporal-nasal and dorso-ventral axes. For example, M- and S-opsin cones are arranged in a specific dorso-ventral gradient 12. Therefore, it is essential to ensure proper orientation of the eye throughout tissue preparation and sectioning in order to perform comparative IHC. We use a cauterizer to place a reference mark on the eye prior to dissection (Figure 2A, D) so that we can orient the eye cup along its dorsal-ventral axis during the embedding phase, and therefore obtaining perfectly transverse sections. We place the burn mark on the dorso-temporal part of the eye, by touching the cornea for a split second with the cauterizer. This will allow to slightly burn the cornea while avoiding piercing a hole into it. During the eye cup dissection, we make a small incision, at the burn mark, perpendicular to border of the cornea, using the micro knife (Figure 2B). To facilitate the dorso-ventral orientation of the block and minimize orientation adjustments during sectioning, we suggest orienting the posterior eye cup in the cryomold, with the burn mark facing the bottom of the cryomold and the opening of the cornea perfectly parallel of the cryomold's right side (Figure 3A, B). The frozen block will be installed with the bottom of the block facing toward the blade in the cryostat, the eye cup perpendicular to the blade, in a dorso-ventral orientation (Figure 3c).
Reliable retina IHC starts with optimal, adapted, and reproducible dissections and tissue processing. The method outlined here optimally preserves retinal structure and integrity while minimizing autofluorescence and epitope masking that can compromise fluorescent IHC. Following this protocol will ensure that you have reproducible high-quality data.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by funds from NIH (RO1-GMO97327) and the University Research Foundation (UPenn). We especially thank Svetlana Savina for her help in developing the immunohistochemistry protocol, Gordon Ruthel (University of Pennsylvania) and the Penn Vet Imaging Core for assistance with microscopy and Leslie King, Ph.D. for critical reading this manuscript.
Materials
| 6qt. Stainless Steel Beaker (185 x 218mm) | Gilson | #MA-48 | For liquid nitrogen (Figure 1E) |
| Aluminum foil | |||
| Cauterizer | Bovie | #AA01 | To mark eye orientation (Figure 1A) |
| Curved forceps, Dumont #5/45 tweezers, 45-degrees bent | Electron Microscopy Sciences | #72703-D | For mouse eye enucleation and maintenance (Figure 1A) |
| Curved scissors, Vannas Spring Scissors – 2.5mm Cutting Edge | Fine Science Tools | #15002-08 | To circumferentially cut the cornea (Figure 1B) |
| Dental wax-coated 35mm dissection dish | For eye cup dissection (Figure 1C) | ||
| Dissecting microscope | Leica, Houston, USA | MZ12.5 High-performance stereomicroscope | |
| Micro Knives – Plastic Handle | Fine Science Tools | #10315-12 | To make a small incision at the burn mark (Figure 1A) |
| Pink dental wax | Electron Microscopy Sciences | #72660 | For coating dissection dish |
| Slide Rack and Coverplate | Ted Pella | #36107 | For retinal IHC (Figure 1G) |
| Stainless Steel Beaker (89 x 114mm) | Gilson | #MA-40 | For isopentane bath (Figure 1E) |
| Styrofoam box | For insulated cooler | ||
| Thin forceps Dumont #5 | Fine Science Tools | #11254-20 | To remove the cornea and extract the lens (Figure 1B) |
| Tissue-Tek cryomold (10mm x 10mm x 5mm) | Electron Miscroscopy Sciences | #62534-10 | Mold for OCT embedding |
| Buffers and Reagents | Company | Catalog Number | Comments |
| Blocking solution | 2% Normal Horse Serum, 1.5% Cold Fish skin Gelatin (at 40-50% in H2O, cat #G7765), 5% bovine serum albumin (cat #AK1391-0100) in permeabilization buffer. | ||
| Gelvatol (anti-fading mounting media) | Under the fume hood, dissolve 0.337g DABCO (Sigma cat #D2522-25G) in 10mL Fluoromount G (Fisher cat #OB100-01). Adjust to pH 8-8.5 with 12N HCl (~ 5 drops). Store in amber drop bottle at 4°C (8). | ||
| Hoechst 33342 1000x Stock | Thermo Scientific | #62249 | 1 mg/ml in PBS (1000x). Aliquot and store in dark, at 4°C for up to 1 year or at -20°C for long term storage. Prepare fresh at 1 ug/mL (1x) |
| Isopentane, 99% | GFS Chemicals | #2961 | |
| Liquid Nitrogen | |||
| Paraformaldehyde (PFA) in PBS | Electron Microscopy Sciences | #15710 | Prepared fresh 4% PFA from 16% PFA stock. Store the remaining 16% PFA at 4°C in the dark. |
| Permeabilization solution | PBS + 0.25% Triton-X (AMRESCO cat #0694-1L) + 0.05% NaN3. Prepare fresh. | ||
| Phosphate-buffered saline (PBS) | Bioworld | #41620015-20 | 0.02 g/L KCl, 0.02 g/L KH2PO4, 0.8 g/L NaCl, 0.216 g/L Na2HPO4 , pH 7.4. Prepared from 10x stock |
| Sucrose solutions | Fisher Scientific | #S-5-500 | Dissolve the appropriate amount of sucrose in 37°C PBS (takes ~15 min to dissolve). May be stored for a few days at 4°C. |
| Tissue-Tek OCT-compound | Sakura | #4583 | |
| Antibodies | Company | Catalog Number | Comments |
| anti-calbindin | Sigma-Aldrich | C8666 | To label horinzotal cells |
| anti-GAD65 | Chemicon | AB5082 | To label GABAergic amacrine cells |
| anti-GS | BD Transduction | 610517 | To label Müller cells |
| anti-rhodopsin | Millipore | MAB5316 | To label rods |
Riferimenti
- Coons, A. H. Labelled antigens and antibodies. Annual Review of Microbiology. 8, 333-352 (1954).
- Coons, A. H. Fluorescent antibodies as histochemical tools. Federation Proceedings. 10 (2), 558-559 (1951).
- Coons, A. H., Kaplan, M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. Journal of Experimental Medicine. 91 (1), 1-13 (1950).
- Chatterjee, S. Artefacts in histopathology. Journal of Oral Maxillofacial Pathology. 18 (Suppl 1), S111-S116 (2014).
- Hartz, P. H. Frozen sections from Bouin-fixed material in histopathology. Stain Technology. 20, 113 (1945).
- Benerini Gatta, L., et al. Application of alternative fixatives to formalin in diagnostic pathology. European Journal of Histochemistry. 56 (2), e12 (2012).
- Tokuyasu, K. T. Application of cryoultramicrotomy to immunocytochemistry. Journal of Microscopy. 143 (Pt 2), 139-149 (1986).
- Imai, H., et al. Molecular properties of rhodopsin and rod function. Journal of Biological Chemistry. 282 (9), 6677-6684 (2007).
- Lee, J. W., Lim, M. Y., Park, Y. S., Park, S. J., Kim, I. B. Reexamination of Dopaminergic Amacrine Cells in the Rabbit Retina: Confocal Analysis with Double- and Triple-labeling Immunohistochemistry. Experimental Neurobiology. 26 (6), 329-338 (2017).
- Bringmann, A., Grosche, A., Pannicke, T., Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Muller) Cells. Frontiers in Endocrinology (Lausanne). 4, 48 (2013).
- Nakhai, H., et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 134 (6), 1151-1160 (2007).
- Applebury, M. L., et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 27 (3), 513-523 (2000).

